Answered step by step
Verified Expert Solution
Question
1 Approved Answer
answer please ! Question 8 (1 point) Consider the following reaction: 4NH3(g) + 3O2(g) --> 2N2(g) + 6H2O(g) The diagram below represents a mixture of
answer please ! 
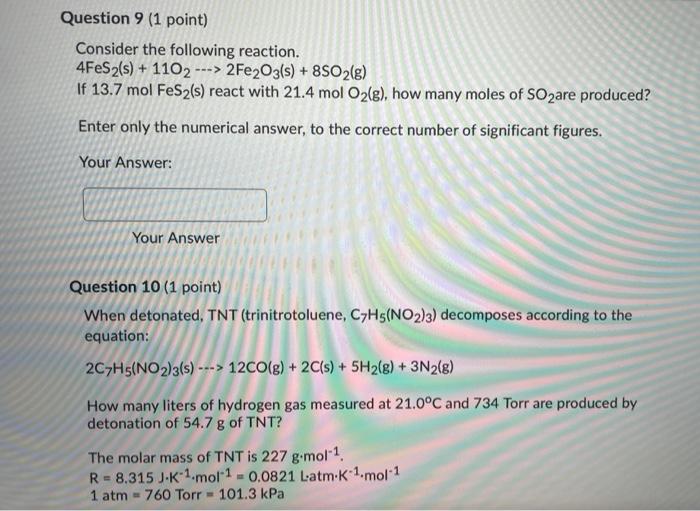
Question 8 (1 point) Consider the following reaction: 4NH3(g) + 3O2(g) --> 2N2(g) + 6H2O(g) The diagram below represents a mixture of 8 moles of NH3(g) and 10 moles of O2(g). ogo 86 28 (1) What is the limiting reactant? Choose from A for NH3(g), B for O2(g), C for N2(g) or D for H2O(g). Type the LETTER of your choice ONLY in Blank #1. (2) How many moles of excess reactant are left over after complete reaction? Enter the numerical value only in Blank #2. Blank # 1 Blank #2 Question 9 (1 point) Consider the following reaction. 4FeS2(s) + 1102 2Fe2O3(s) + 8SO2(g) If 13.7 mol FeS2(s) react with 21.4 mol O2(g), how many moles of SOzare produced? > Enter only the numerical answer, to the correct number of significant figures. Your Answer: Your Answer Question 10 (1 point) When detonated, TNT (trinitrotoluene, C7H5(NO2)3) decomposes according to the equation: 2C7H5(NO2)3(s) ---> 12CO(g) + 2C(s) + 5H2(g) + 3N2(8) How many liters of hydrogen gas measured at 21.0C and 734 Torr are produced by detonation of 54.7 g of TNT? The molar mass of TNT is 227 g.mol-1 R = 8.315 J-K-1.mol-1 -0.0821 Latm.K 1.mol-1 1 atm = 760 Torr - 101.3 kPa 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


