Answered step by step
Verified Expert Solution
Question
1 Approved Answer
answer questions based on graph 6. From the slope andior intercepts of the line calculation both the enthalpy ( (kJ mol 1 ) and entropy
answer questions based on graph 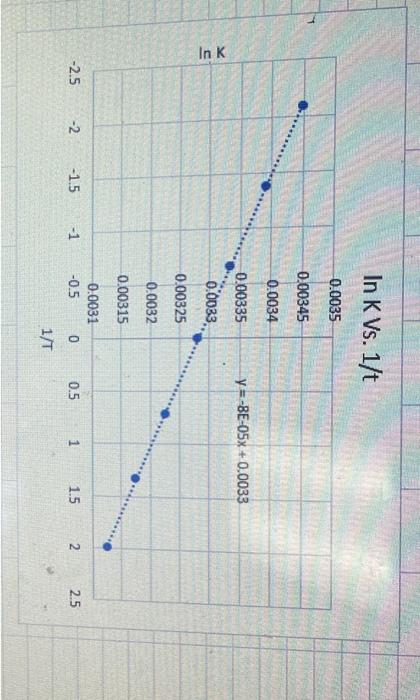


6. From the slope andior intercepts of the line calculation both the enthalpy ( (kJ mol 1 ) and entropy (Jmol 1K1) for the process. Use Linest to report your answers with the error, proper number of significant figures and the appropriate units: 7. Calculate the meling temperature, Tm, of the protein. At the meiting temperature, under standard conditions G=0. 8. Calculate G at 292K and 318K and predict the most stable form of the protein at these temperatures 1. Completed assignment with spreadsheet info along with properly labeled and scaled graph with equation of best-fit line, intercept and correlation coefficient Calculation of both the enthalpy and entropy for unfolding process, along with errors, proper number of significant figures and proper units 2. Calculation the melting temperature of the protein (in K ) to 3 significant figures 3. Determination of the stability of the protein at 292K and 318K 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


