Answered step by step
Verified Expert Solution
Question
1 Approved Answer
answer step 1, and step 2 please Methane (CH4) at 298 K, 1 atm enters a furnace operating at steady state and burns completely with
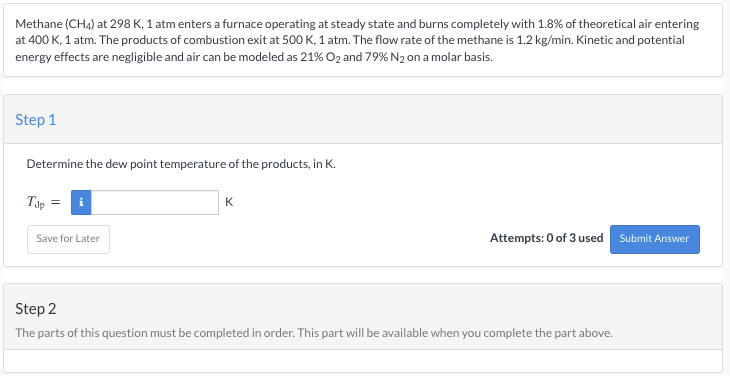
answer step 1, and step 2 please
Methane (CH4) at 298 K, 1 atm enters a furnace operating at steady state and burns completely with 1.8% of theoretical air entering at 400 K, 1 atm. The products of combustion exit at 500 K, 1 atm. The flow rate of the methane is 1.2 kg/min. Kinetic and potential energy effects are negligible and air can be modeled as 21% O2 and 79% N2 on a molar basis. Step 1 Determine the dew point temperature of the products, in K. Tip = K Save for Later Attempts: 0 of 3 used Submit Answer Step 2 The parts of this question must be completed in order. This part will be available when you complete the part aboveStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


