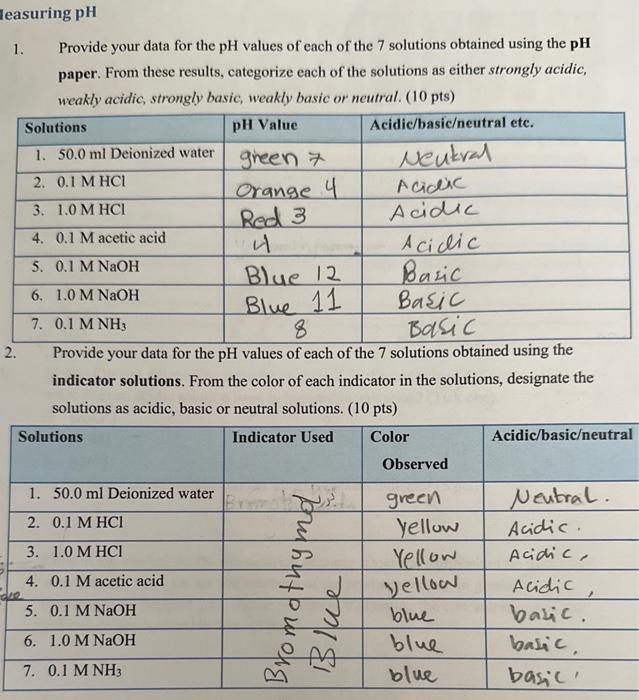
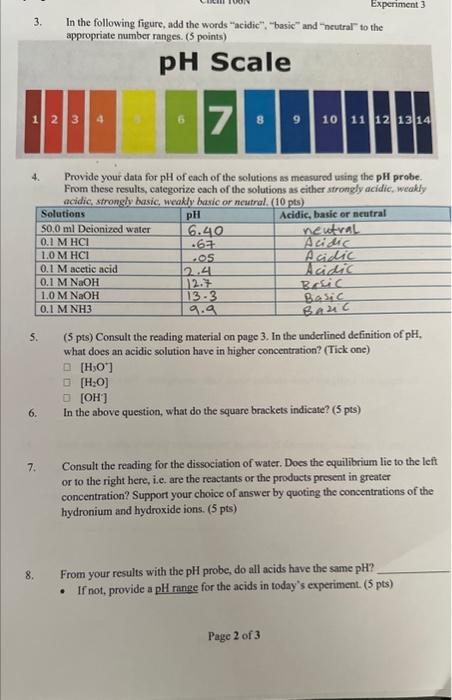
easuring pH 1. Provide your data for the pH values of each of the 7 solutions obtained using the pH paper. From these results, categorize each of the solutions as either strongly acidic, weakly acidic, strongly basic, weakly basic or neutral. (10 pts) Provide your data for the pH values of each of the 7 solutions obtained using the indicator solutions. From the color of each indicator in the solutions, designate the solutions as acidic, basic or neutral solutions. (10 pts) 3. In the following figure, add the words "acidic". "basic" and "ncutral" to the appropriate number ranges. ( 5 points) 4. Provide your data for pH of each of the solutions as measured using the pH probe. From these results, categorize each of the solutions as either strongly acidic, weakly acidie stmonolv hasie weakly havin nr meutmal (10 nts) 5. (5 pts) Consult the reading material on page 3. In the underlined definition of pH, what does an acidic solution have in higher concentration? (Tick one) [H3O+][H2O][OH] 6. In the above question, what do the square brackets indicate? ( 5 pts) 7. Consult the reading for the dissociation of water. Does the equilibrium lie to the left or to the right here, i.e. are the reactants or the products present in greater concentration? Support your choice of answer by quoting the concentrations of the hydronium and hydroxide ions. ( 5 pts) 8. From your results with the pH probe, do all acids have the same pH ? - If not, provide a pll range for the acids in today's experiment. ( 5 pts) 9. For equal concentrations of HCl and acetic acid: (5pts) - Which has the lower pH value? - Therefore, which has the higher concentration of Hj ? 10. From your results with the pH probe, do all bases have the same pH ? - If not, provide a pH range for the bases in today's experiment. ( 5 pts) 11. For equal concentrations of two bases, NaOH and NH3,(5pts) - Which one has a higher pH ? - Therefore, which has the lower concentration of H3O+? 12. In the table below, calculate the pH values of each of the following strong acud aqueous solutions (5 ntel 13. Consider your own pH data and answer the following: ( 5pts) - What does a pH less than water (pH=7) indicate about the [H3O+]in that solution? - What does a pH value greater than water indicate about the [H3O+]in solution? 14. Rank each method of pH measurement in order of decreasing certainty. (5pts) a) b) c)