Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Answer these following questions below based on the data that is provided also below Complete the conclusion and question using provided data Conclusion Be concise
Answer these following questions below based on the data that is provided also below 
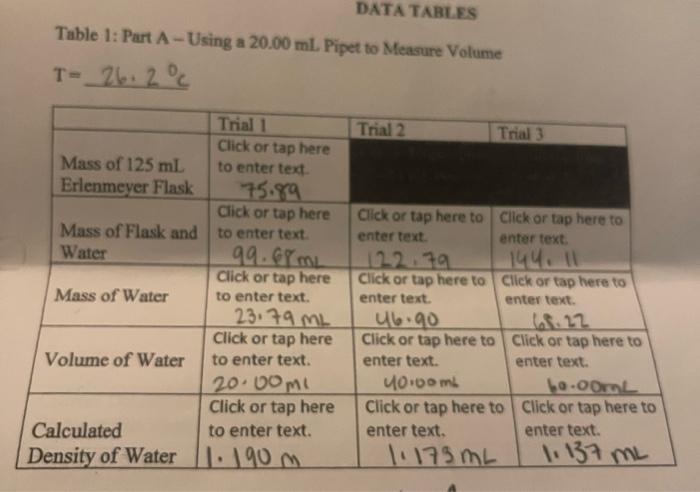
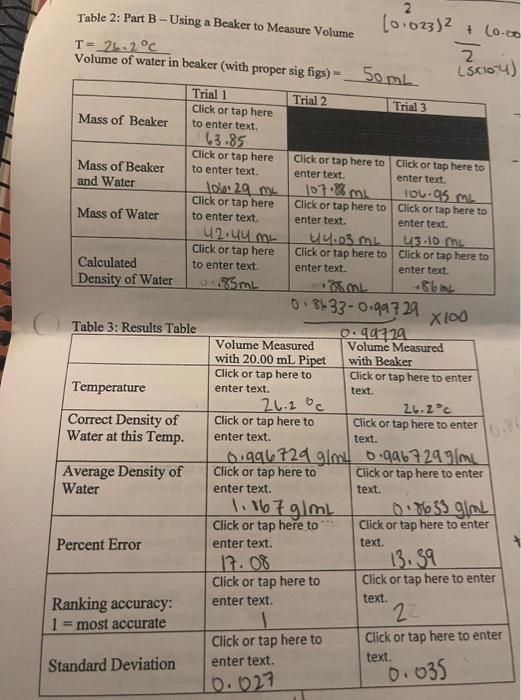



Complete the conclusion and question using provided data
Conclusion Be concise but thorough enough that whoever reads your conclusion knows how you did your experiment and the outcome WITH NUMERICAL. VALUES. The reader should also know how to improve the experiment if they should try to reproduce your results. Questions fake sure you have already answered the Trends nd Sources of Error before answering the llowing questions. 1. Suppose you are required to measure out exactly 20.000.02mL of a liquid. Which piece of glassware would you use? Base your answer on the piece of glassware that you found to be most accurate and/or precise. 2. In mL what is the volume of liquid in the graduated cylinder pictured on the right (to the correct number of significant figures)? 3. For the graduated cylinder pictured on the right, where would you expect it to rank as far as accuracy and precision in comparison with the other two pieces of glassware you used in this experiment. Why? 4. You accidentally blow out all of the water (without noticing) from your "To Deliver" pipet. How would this affect your calculated density (higher or lower)? Explain. 5. In Part B, why did you have to initially weigh a dry beaker, but for the subsequent measurements, the beaker did not have to be dried? 6. We could have performed this experiment with ethylene glycol (d=1.10g/mL) instead. If you measured out equal volumes of ethylene glycol and water, which would have the greater mass? Explain Table 1: Part A - Using a 20.00mL Pipet to Measure Volume T=2620c T=242C Volume of water in beaker (with proper sie fios) =5. 1.510 0.85330.99729100 Conclusion Be concise but thorough enough that whoever reads your conclusion knows how you did your experiment and the outcome WITH NUMERICAL. VALUES. The reader should also know how to improve the experiment if they should try to reproduce your results. Questions fake sure you have already answered the Trends nd Sources of Error before answering the llowing questions. 1. Suppose you are required to measure out exactly 20.000.02mL of a liquid. Which piece of glassware would you use? Base your answer on the piece of glassware that you found to be most accurate and/or precise. 2. In mL what is the volume of liquid in the graduated cylinder pictured on the right (to the correct number of significant figures)? 3. For the graduated cylinder pictured on the right, where would you expect it to rank as far as accuracy and precision in comparison with the other two pieces of glassware you used in this experiment. Why? 4. You accidentally blow out all of the water (without noticing) from your "To Deliver" pipet. How would this affect your calculated density (higher or lower)? Explain. 5. In Part B, why did you have to initially weigh a dry beaker, but for the subsequent measurements, the beaker did not have to be dried? 6. We could have performed this experiment with ethylene glycol (d=1.10g/mL) instead. If you measured out equal volumes of ethylene glycol and water, which would have the greater mass? Explain Table 1: Part A - Using a 20.00mL Pipet to Measure Volume T=2620c T=242C Volume of water in beaker (with proper sie fios) =5. 1.510 0.85330.99729100 Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


