Question
Answer this: A reaction is said to have been completed if one of the reactants is completely consumed by the reaction. In this experiment, sodium


Answer this:
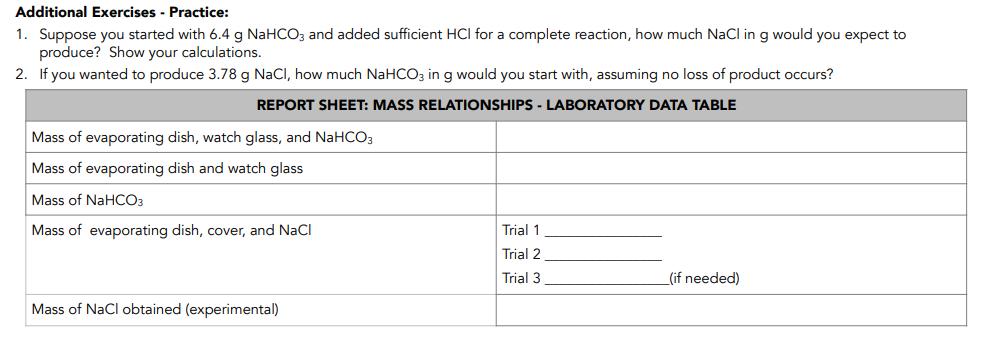
A reaction is said to have been completed if one of the reactants is completely consumed by the reaction. In this experiment, sodium bicarbonate (baking soda) is made to react with hydrochloric acid to produce sodium chloride according to the reaction: NaHCO3 +HCI - NaCI + H2O + CO2(g) You will use an accurately measured amount of NaHCOz and add enough HCI until the bicarbonate is completely used up. You will isolate the product, NaCl, from the other products and determine its mass. This is the actual yield of the reaction. The theoretical yield can be calculated by using the mass relationships in the balanced chemical equation above. The percentage yield can be determined from the ratio of the actual yield to the theoretical yield.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Introduction to Operations Research
Authors: Frederick S. Hillier, Gerald J. Lieberman
10th edition
978-0072535105, 72535105, 978-1259162985
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



