Answered step by step
Verified Expert Solution
Question
1 Approved Answer
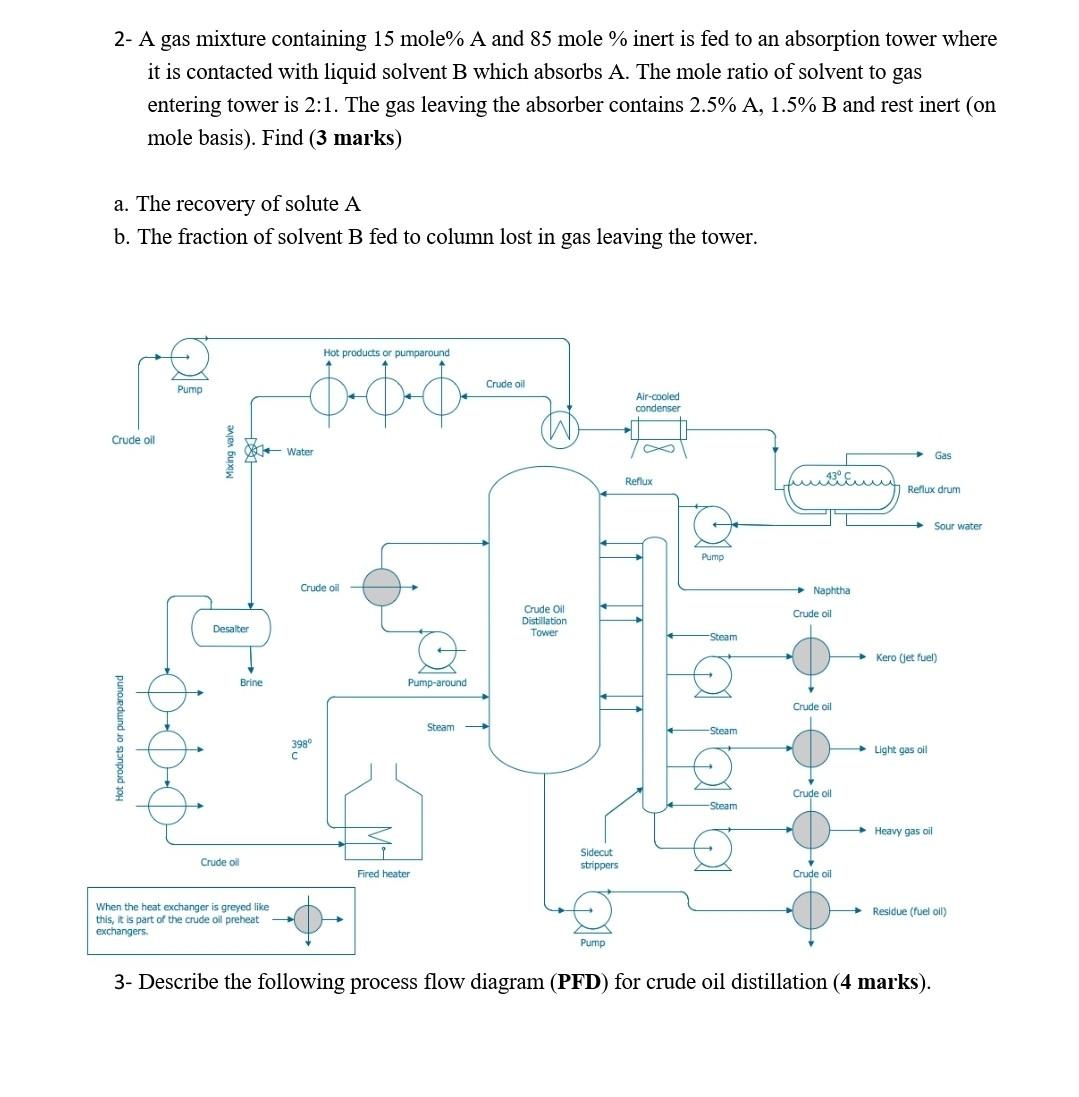
answer this two questions pls 2- A gas mixture containing 15 mole% A and 85 mole % inert is fed to an absorption tower where
answer this two questions pls
2- A gas mixture containing 15 mole\% A and 85 mole % inert is fed to an absorption tower where it is contacted with liquid solvent B which absorbs A. The mole ratio of solvent to gas entering tower is 2:1. The gas leaving the absorber contains 2.5%A,1.5%B and rest inert (on mole basis). Find (3 marks) a. The recovery of solute A b. The fraction of solvent B fed to column lost in gas leaving the tower. s- Describe the rollowing process Ilow alagram (YFW) for cruae oll alstination (4 marks)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


