Answers needed.
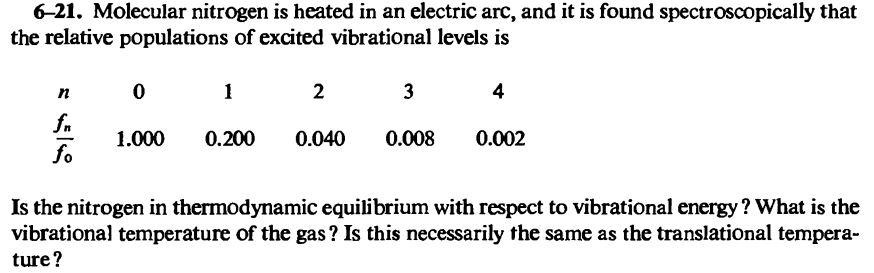
6-21. Molecular nitrogen is heated in an electric arc, and it is found spectroscopically that the relative populations of excited vibrational levels is n O 2 3 fn 1.000 0.200 0.040 0.008 0.002 Is the nitrogen in thermodynamic equilibrium with respect to vibrational energy ? What is the vibrational temperature of the gas ? Is this necessarily the same as the translational tempera- ture?13. You are a scientist at Merck and your annual progress report is coming up soon. Unfortunately, you have gotten nothing done over the past year and are running out of time. Your target is a large enzyme whose crystal structure has been previously screened in earlier drug design campaigns, and molecular dynamics simulations have been run for this enzyme. What would be the best course of action to take in the limited time you have left? A. Run a standard precision (SP) virtual screen on the crystal structure B. Run several XP virtual screens on previously-obtained conformations obtained from molecular dynamics simulations run on the crystal structure C. Run a SP virtual screen on conformations obtained from molecular dynamics simulations run on the crystal structure D. Begin high-throughput screening on the enzyme E. Start with a high-throughput screen on the enzyme, followed by a SP virtual screen on conformations obtained from molecular dynamics simulations run on the crystal structureEnter Name of reactants and products: Did Precipitate form in the simulation? lead (II) nitrate + sodium sulfide Balanced Total equation (Molecular equation) if so, what is the name of the precipitate? E Net lanie equation: What color was the precipitate? Net ionic equation: Name of reactants and products: Did Precipitate form in the simulation? copper(II) nitrate + sodium iodide > Balanced Total equation (Molecular equation) If so, what is the name of the precipitate" F Net fonic equation: What color was the precipitate? Net ionic equation: Name of reactants and products: Did Precipitate form in the simulation? Balanced Total equation (Molecular equation) If ser, what is the name of the Ca(NO, )(ag) + Nal(ag) > precipitate? G Net fonic equation: What color was the precipitate? Net ionic equation: Page | 54. One of the computational chemical methods used to determine the energy of a compound is Molecular Mechanics. Molecular mechanical equations are stated as follows: E= [ko(T-To)? + [ko(0-8.)? + 41+ cos(nt - ] lori + EET 919 1 + + (a) Explain the meaning of each term in the equation above so that it can be explained the contribution of each component determining the amount of energy. (b) Describe the strengths and weaknesses of molecular mechanics methods in determining the properties of compounds