Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Any help is greatly appreciated! Part 1: Find mixture and individual TLVs resulting from a liquid mixture comprising 40% methanol, 30% methyl acetate, and 30%
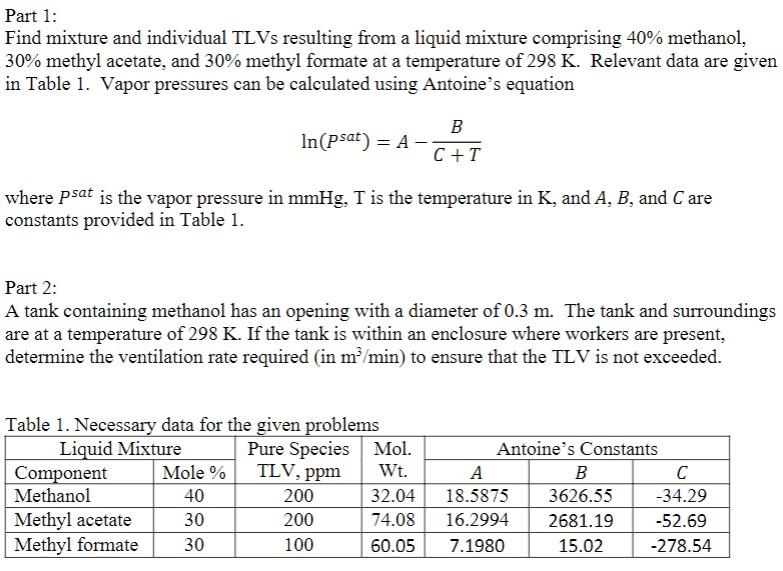
Any help is greatly appreciated!
Part 1: Find mixture and individual TLVs resulting from a liquid mixture comprising 40% methanol, 30% methyl acetate, and 30% methyl formate at a temperature of 298 K. Relevant data are given in Table 1. Vapor pressures can be calculated using Antoine's equation B In(psat) = A - C+T where psat is the vapor pressure in mmHg, T is the temperature in K, and A, B, and Care constants provided in Table 1. Part 2: A tank containing methanol has an opening with a diameter of 0.3 m. The tank and surroundings are at a temperature of 298 K. If the tank is within an enclosure where workers are present, determine the ventilation rate required (in m/min) to ensure that the TLV is not exceeded. TLV, ppm Table 1. Necessary data for the given problems Liquid Mixture Pure Species Mol. Component Mole % Wt. Methanol 40 200 32.04 Methyl acetate 30 200 74.08 Methyl formate 30 100 60.05 Antoine's Constants B 18.5875 3626.55 -34.29 16.2994 2681.19 -52.69 7.1980 15.02 -278.54Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


