Question
Any help is greatly appreciated! Please answer in full details! Using the Van Der Waals equation and ideal gas equation , 1a) Graph the non
Any help is greatly appreciated! Please answer in full details!
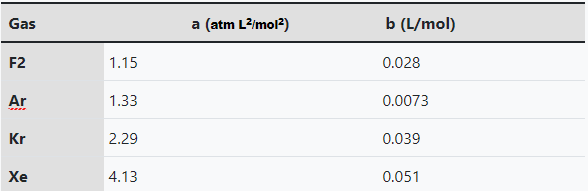
Using the Van Der Waals equation and ideal gas equation,
1a) Graph the non ideal and ideal gas as a pressure vs volume graph with volumes restricted from 1.0 L - 10.0 L at a temperature of 25 K using all gasses listed above.
1b) From the graph. Identify which gas contains a maximum deviation and smallest deviation that transpires between the non ideal and ideal gas and the percent deviation (hint: determine volume and temperature and proceed from there).
1c) Does the values calculated prove the ideal gas equation? How?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


