any help with this assignment is greatly appreciated
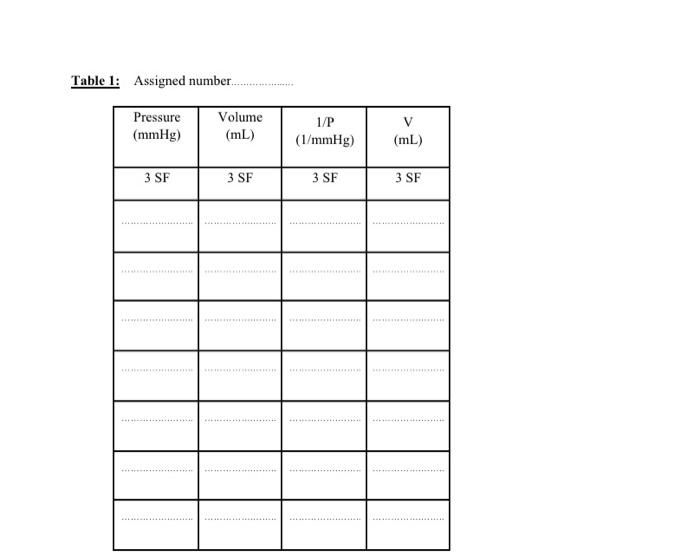

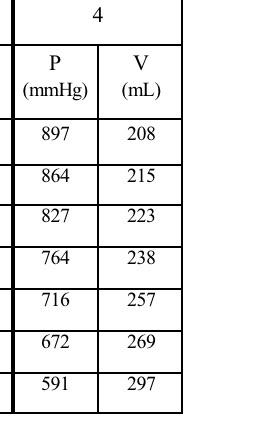
4 P (mmHg) V (mL) 897 208 864 215 827 223 764 238 716 257 672 269 591 297 Data sheet: Complete the data sheet (Table I below) based on your assigned set of measurements (1 - 4). Apply Significant Figure (SF) rules to all the calculations. 1. Copy the assigned measurements and calculate the missing values to the correct SF. 2- For the values of 1/P, round off to 3 SF in scientific notation. 3. Using MS Excel, create two graphs (Graph 1: V vs. P; Graph 2: V vs. 1/P) to graphically analyze these data between the gas volume and its pressure, and to verify Boyle's law. Be sure to properly label each axis. 4- Based on these graphs, select and include on the graph sheets the proper trend lines to maximize the R values, along with the equations relating the volume vs. pressure, and volume vs. 1/pressure. Table 1: Assigned number... Pressure (mmHg) Volume (mL) 1/P (1/mmHg) V (mL) 3 SF 3 SF 3 SF 3 SF Objectives: 1. To examine and verify the relationships between the pressure, volume, and temperature of a gas as stated in Boyle's Law and Gay-Lussac's Law. 2. To construct graphs using experimental data to illustrate these relationships: For Boyle's Law, pressure and volume are inversely related. For Guy Lussac's Law, pressure and temperature are directly related. Discussion: In 1662, Robert Boyle, born in Ireland, studied the relationship between the pressure and volume of a sample of gas. His work led to the generalization now known as Boyle's Law, which states: at constant temperature, the volume of a given quantity of gas varies inversely with its pressure. Stated another way: as volume increases, pressure decreases and vice versa. At the time Boyle was conducting his experiments, the thermometer had not yet been invented. Thus, his original statement mentioned only that the "hotness" of the gas must be held constant. In 1802, nearly 80 years after the invention of the thermometer, Joseph Louis Gay-Lussac published his work on the relationship between the kinetic energy of a gas and its pressure. He stated: if you increase the kinetic energy or speed of the molecules by increasing their temperature, the force of the molecules hitting the inside of the container increases and this increases the pressure. This means that as the temperature increases, the pressure increases, and vice versa. However, Gay-Lussac based his work on the earlier findings of Guillaume Amontons. who, between 1700 and 1702, discovered the relationship between the pressure and temperature of a fixed mass of gas kept at a constant volume. This relationship is often referred to as Gay- Lussac's Law or Avontans' Law of Pressure-Temperature. There are 2 parts to this virtual Experiment: Part A refers to Boyle's Law and Part B to Gay- Lussac's Law. You must complete the two parts