Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The volume of a certain lake V=1.0 107m3, input water quantity of tributary Qin=0.5 108m3/a, input a certain pollutant concentration of Cin=3mg/L. The initial concentration
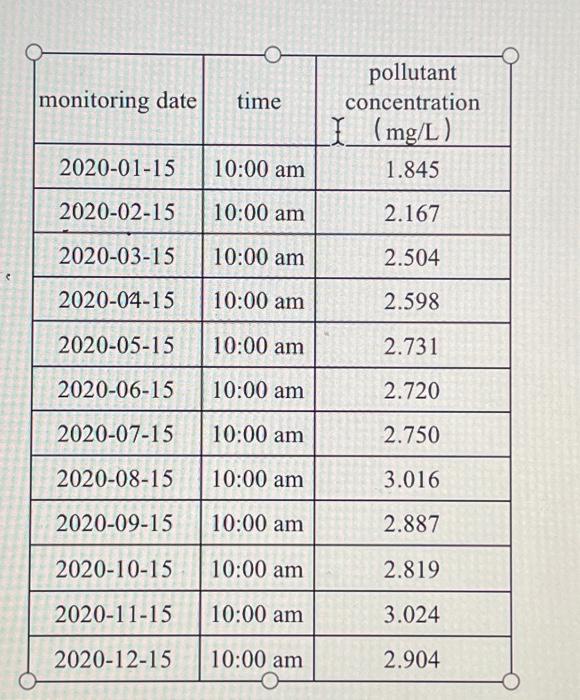
The volume of a certain lake V=1.0 107m3, input water quantity of tributary Qin=0.5 108m3/a, input a certain pollutant concentration of Cin=3mg/L. The initial concentration of this pollutant in the lake at 0:00 on January 1, 2020, C0=1.5mg/L, and the attenuation of the pollutant in the lake follows a first-order reaction kinetics law. Sampling and monitoring of lake outflow were conducted, and the results are shown in the table on the right.
(1) Please construct a zero dimensional water quality model for the lake for this pollutant. Using optimization methods and monitoring data from 1, 2, 4, 5, 7, 8, 10, and 11 months, determine the value of the pollutant reaction rate constant k (1/a). (Reminder: According to preliminary estimates, the value of k is between 0 and 0.25/a.)
(2) Using observation data from March, June, September, and December, select error based evaluation indicators for model validation. Compare the error levels between the parameter estimation stage and the model validation stage, and provide validation conclusions.
(3) Predict the concentration of pollutants in the lake at 0:00 on July 1, 2023.
(4) We found that there were issues with the observation data for August and November. After removing these two data points, we conducted parameter estimation and validation again. Has the conclusion changed? Discuss it.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


