Answered step by step
Verified Expert Solution
Question
1 Approved Answer
anyone can help me answer asap please In a certain functional beverage, one of the main attributes that determine the shelf life of the beverage
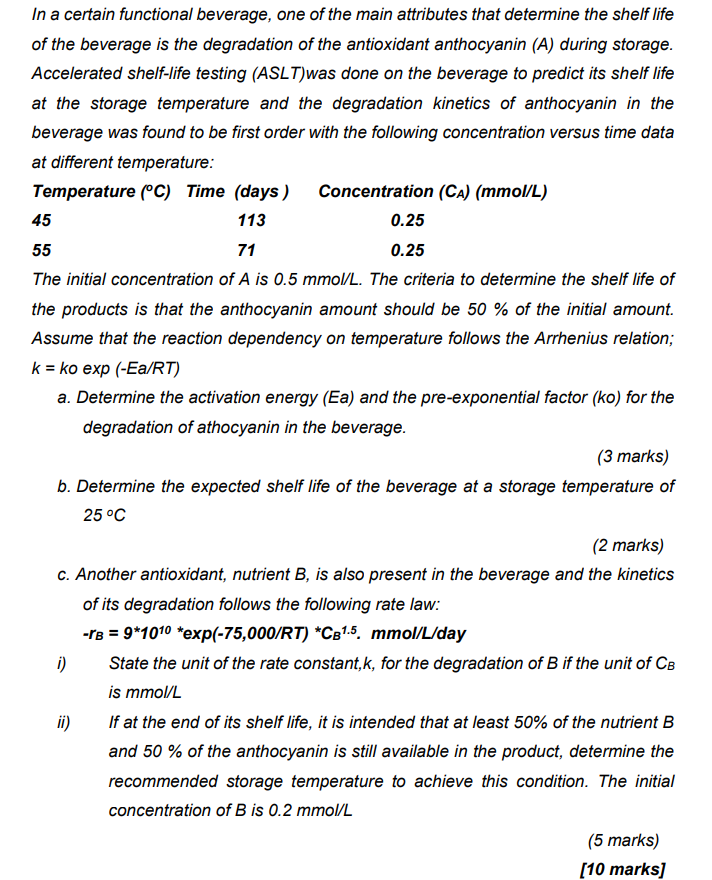
anyone can help me answer asap please
In a certain functional beverage, one of the main attributes that determine the shelf life of the beverage is the degradation of the antioxidant anthocyanin (A) during storage. Accelerated shelf-life testing (ASLT)was done on the beverage to predict its shelf life at the storage temperature and the degradation kinetics of anthocyanin in the beverage was found to be first order with the following concentration versus time data at different temperature: Temperature (C) Time (days) Concentration (CA) (mmol/L) 45 113 0.25 55 71 0.25 The initial concentration of A is 0.5 mmol/L. The criteria to determine the shelf life of the products is that the anthocyanin amount should be 50 % of the initial amount. Assume that the reaction dependency on temperature follows the Arrhenius relation; k = ko exp (-Ea/RT) a. Determine the activation energy (Ea) and the pre-exponential factor (ko) for the degradation of athocyanin in the beverage. (3 marks) b. Determine the expected shelf life of the beverage at a storage temperature of 25 C (2 marks) c. Another antioxidant, nutrient B, is also present in the beverage and the kinetics of its degradation follows the following rate law: -1B = 9*1010 *exp(-75,000/RT) *C81.5. mmol/L/day i) State the unit of the rate constant,k, for the degradation of B if the unit of CB is mmol/L ii) If at the end of its shelf life, it is intended that at least 50% of the nutrient B and 50 % of the anthocyanin is still available in the product, determine the recommended storage temperature to achieve this condition. The initial concentration of B is 0.2 mmol/L (5 marks) [10 marks] In a certain functional beverage, one of the main attributes that determine the shelf life of the beverage is the degradation of the antioxidant anthocyanin (A) during storage. Accelerated shelf-life testing (ASLT)was done on the beverage to predict its shelf life at the storage temperature and the degradation kinetics of anthocyanin in the beverage was found to be first order with the following concentration versus time data at different temperature: Temperature (C) Time (days) Concentration (CA) (mmol/L) 45 113 0.25 55 71 0.25 The initial concentration of A is 0.5 mmol/L. The criteria to determine the shelf life of the products is that the anthocyanin amount should be 50 % of the initial amount. Assume that the reaction dependency on temperature follows the Arrhenius relation; k = ko exp (-Ea/RT) a. Determine the activation energy (Ea) and the pre-exponential factor (ko) for the degradation of athocyanin in the beverage. (3 marks) b. Determine the expected shelf life of the beverage at a storage temperature of 25 C (2 marks) c. Another antioxidant, nutrient B, is also present in the beverage and the kinetics of its degradation follows the following rate law: -1B = 9*1010 *exp(-75,000/RT) *C81.5. mmol/L/day i) State the unit of the rate constant,k, for the degradation of B if the unit of CB is mmol/L ii) If at the end of its shelf life, it is intended that at least 50% of the nutrient B and 50 % of the anthocyanin is still available in the product, determine the recommended storage temperature to achieve this condition. The initial concentration of B is 0.2 mmol/L (5 marks) [10 marks]Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


