Answered step by step
Verified Expert Solution
Question
1 Approved Answer
applied chemical engineering 6. Consider a galvanic cell consisting of 5Fe2+(aq)+MnO4(aq)+8H+(aq)5Fe3+(aq)+Mn2+(aq)+4H2O(l) i) Write the oxidation and reduction half-reactions ii) Write the reaction using cell notation.
applied chemical engineering 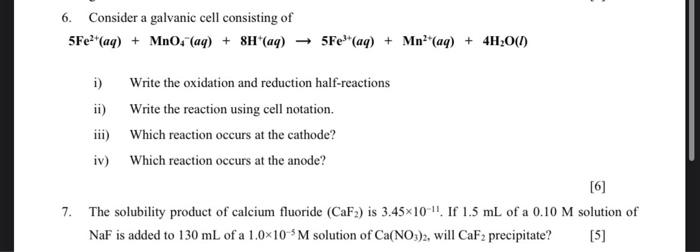
6. Consider a galvanic cell consisting of 5Fe2+(aq)+MnO4(aq)+8H+(aq)5Fe3+(aq)+Mn2+(aq)+4H2O(l) i) Write the oxidation and reduction half-reactions ii) Write the reaction using cell notation. iii) Which reaction occurs at the cathode? iv) Which reaction occurs at the anode? 7. The solubility product of calcium fluoride (CaF2) is 3.451011. If 1.5mL of a 0.10M solution of NaF is added to 130mL of a 1.0105M solution of Ca(NO3)2, will CaF2 precipitate 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


