Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Arrhenius concept Arrhenius acids are substances that, when dissolved in water, increase the concentration of the H+ ion; Arrhenius bases are substances that, when
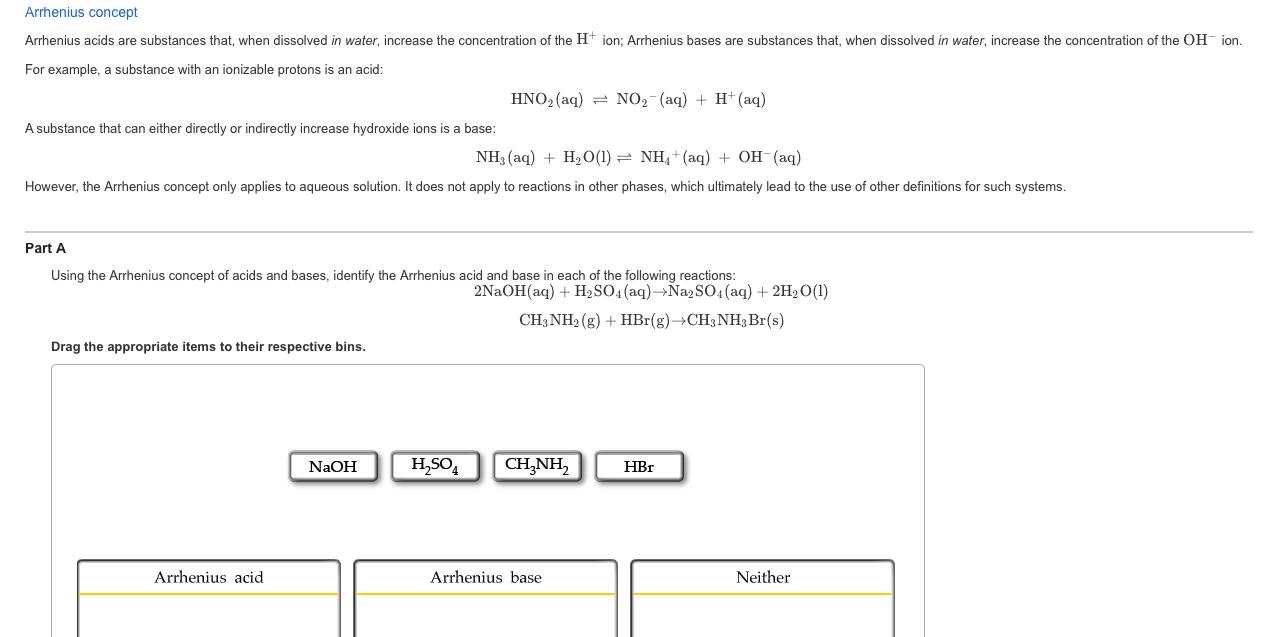
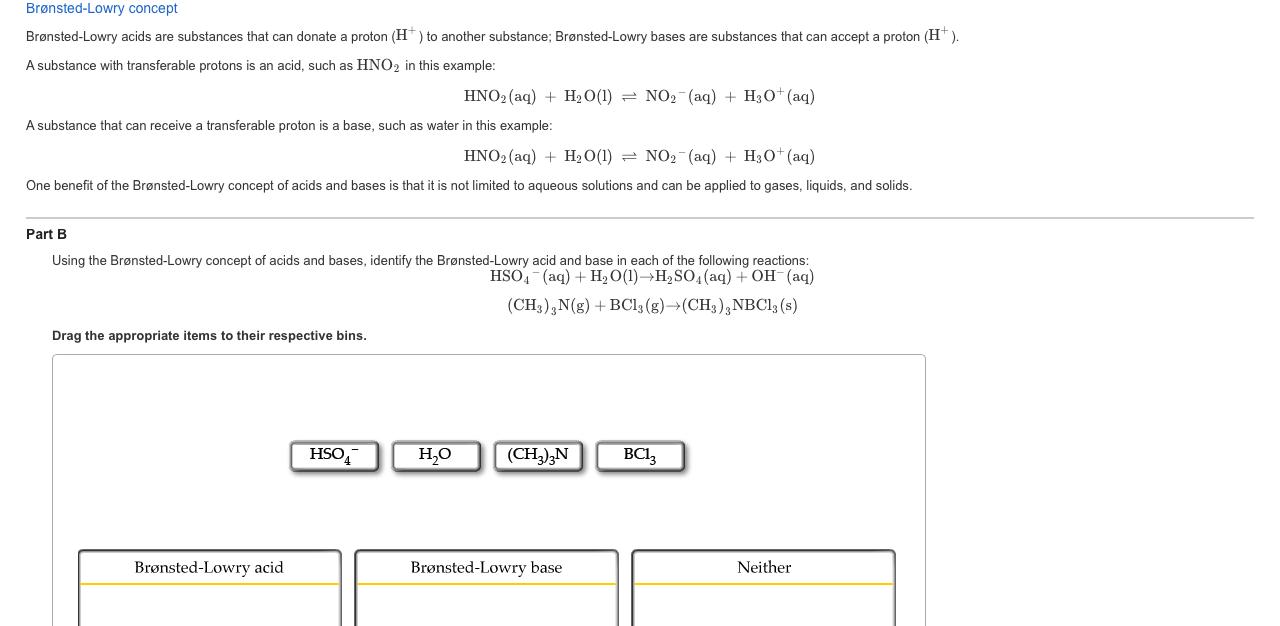
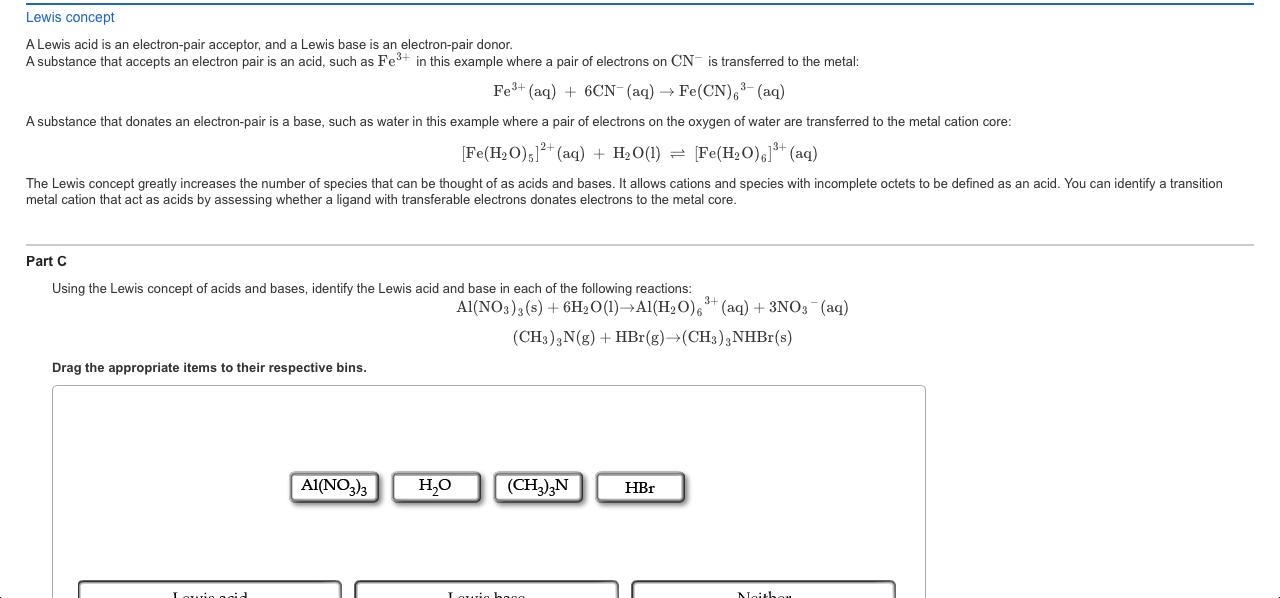
Arrhenius concept Arrhenius acids are substances that, when dissolved in water, increase the concentration of the H+ ion; Arrhenius bases are substances that, when dissolved in water, increase the concentration of the OH ion. For example, a substance with an ionizable protons is an acid: HNO2(aq) NO2 (aq) + H+ (aq) A substance that can either directly or indirectly increase hydroxide ions is a base: NH3(aq) + H2O(1) NH4+(aq) + OH(aq) However, the Arrhenius concept only applies to aqueous solution. It does not apply to reactions in other phases, which ultimately lead to the use of other definitions for such systems. Part A Using the Arrhenius concept of acids and bases, identify the Arrhenius acid and base in each of the following reactions: Drag the appropriate items to their respective bins. 2NaOH(aq) + H2SO4(aq)Na2SO4(aq) + 2H2O(1) CH3NH2(g) + HBr(g) CH3NH3 Br(s) NaOH HSO4 CH3NH2 HBr Arrhenius acid Arrhenius base Neither
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


