Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Arsenic(III) oxide (As,O,) is available in pure form and is a usetul (but carcinogenic) primary standard for oxidizing agents such as MnO. The As,O,
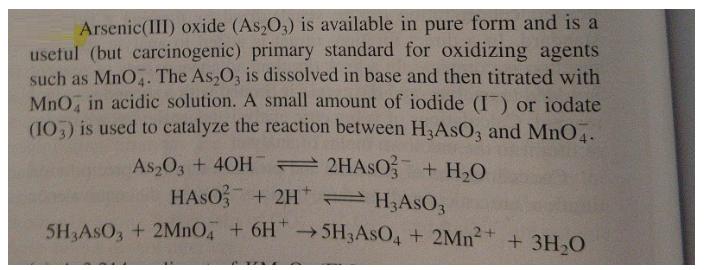

Arsenic(III) oxide (As,O,) is available in pure form and is a usetul (but carcinogenic) primary standard for oxidizing agents such as MnO. The As,O, is dissolved in base and then titrated with MnO, in acidic solution. A small amount of iodide (I) or iodate (10,) is used to catalyze the reaction between H,AsO, and MnO. As O3 + 40H 2HASO + H,O HASO; + 2H H;ASO3 5H AsO, + 2MNO + 6H" 5H3ASO4 + 2Mn? 5H3ASO4 + 2Mn + 3H,O (a) A 3.214-g aliquot of KMNO4 (FM 158.034) was dissolved in 1,000 L of water, heated to cause any reactions with impurities to occur, cooled, and filtered. What is the theoretical molarity of this solution if no MnO, was consumed by impurities? (b) What mass of As,O3 (FM 197.84) would be just sufficient to react with 25.00 mL of the KMNO, solution in part (a)? (e) It was found that 0.146 8 g of As,O, required 29.98 mL of KMnO. solution for the faint color of unreacted MnO4 to appear. In a blank titration, 0.03 ml of MnO, was required to produce enough color to be seen. Calculate the molarity of the permanganate solution
Step by Step Solution
★★★★★
3.44 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


