Answered step by step
Verified Expert Solution
Question
1 Approved Answer
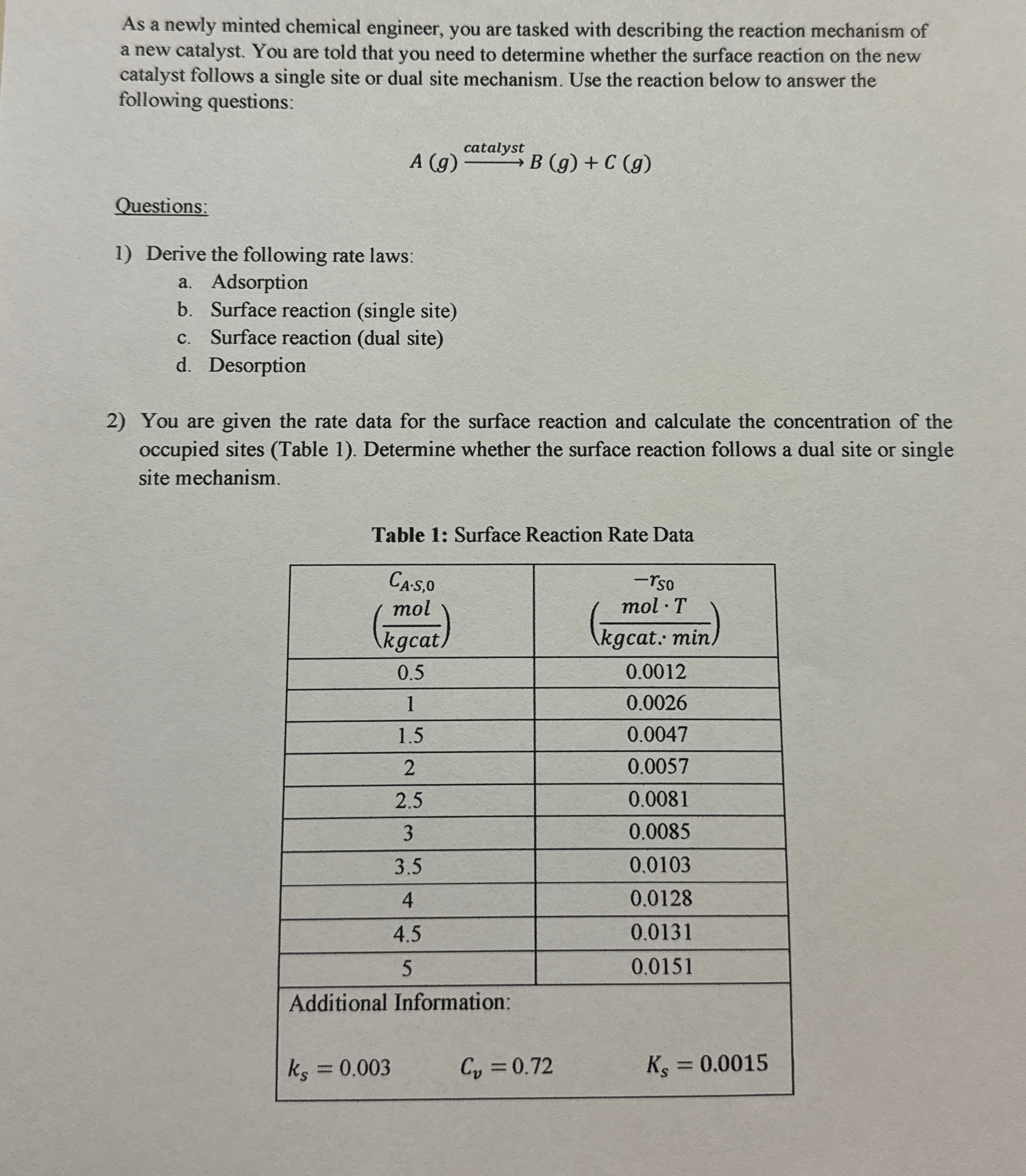
As a newly minted chemical engineer, you are tasked with describing the reaction mechanism of a new catalyst. You are told that you need to
As a newly minted chemical engineer, you are tasked with describing the reaction mechanism of a new catalyst. You are told that you need to determine whether the surface reaction on the new catalyst follows a single site or dual site mechanism. Use the reaction below to answer the following questions:
catalystB
Questions:
Derive the following rate laws:
a Adsorption
b Surface reaction single site
c Surface reaction dual site
d Desorption
You are given the rate data for the surface reaction and calculate the concentration of the occupied sites Table Determine whether the surface reaction follows a dual site or single site mechanism.
Table : Surface Reaction Rate Data
table
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


