Question
As a technician in a large pharmaceutical research firm, you need to produce 150. mL of 1.00 mol L potassium phosphate buffer solution of
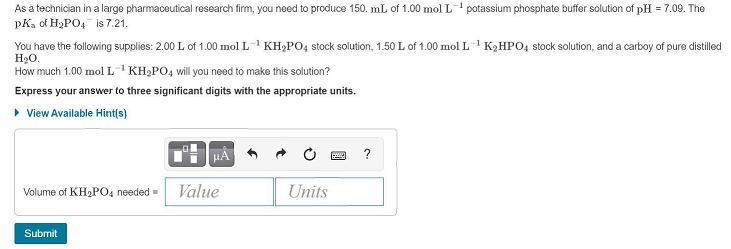
As a technician in a large pharmaceutical research firm, you need to produce 150. mL of 1.00 mol L potassium phosphate buffer solution of pH = 7.09. The pKa of H2PO4 is 7.21. You have the following supplies: 2.00 L of 1.00 mol L1 KH2PO, stock solution, 1.50 L of 1.00 mol L 'K2HPO4 stock solution, and a carboy of pure distilled H2O. How much 1.00 mol L'KH2PO, will you need to make this solution? Express your answer to three significant digits with the appropriate units. View Available Hint(s) ? Volume of KH,PO, needed = Value Units Submit
Step by Step Solution
3.38 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of biochemistry Life at the Molecular Level
Authors: Donald Voet, Judith G. Voet, Charlotte W. Pratt
4th edition
470547847, 978-0470547847
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



