Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The Henderson-Hasselbalch equation in medicine Carbon dioxide (CO2) and bicarbonate (HCO3) concentrations in the bloodstream are physiologically controlled to keep blood pH constant at
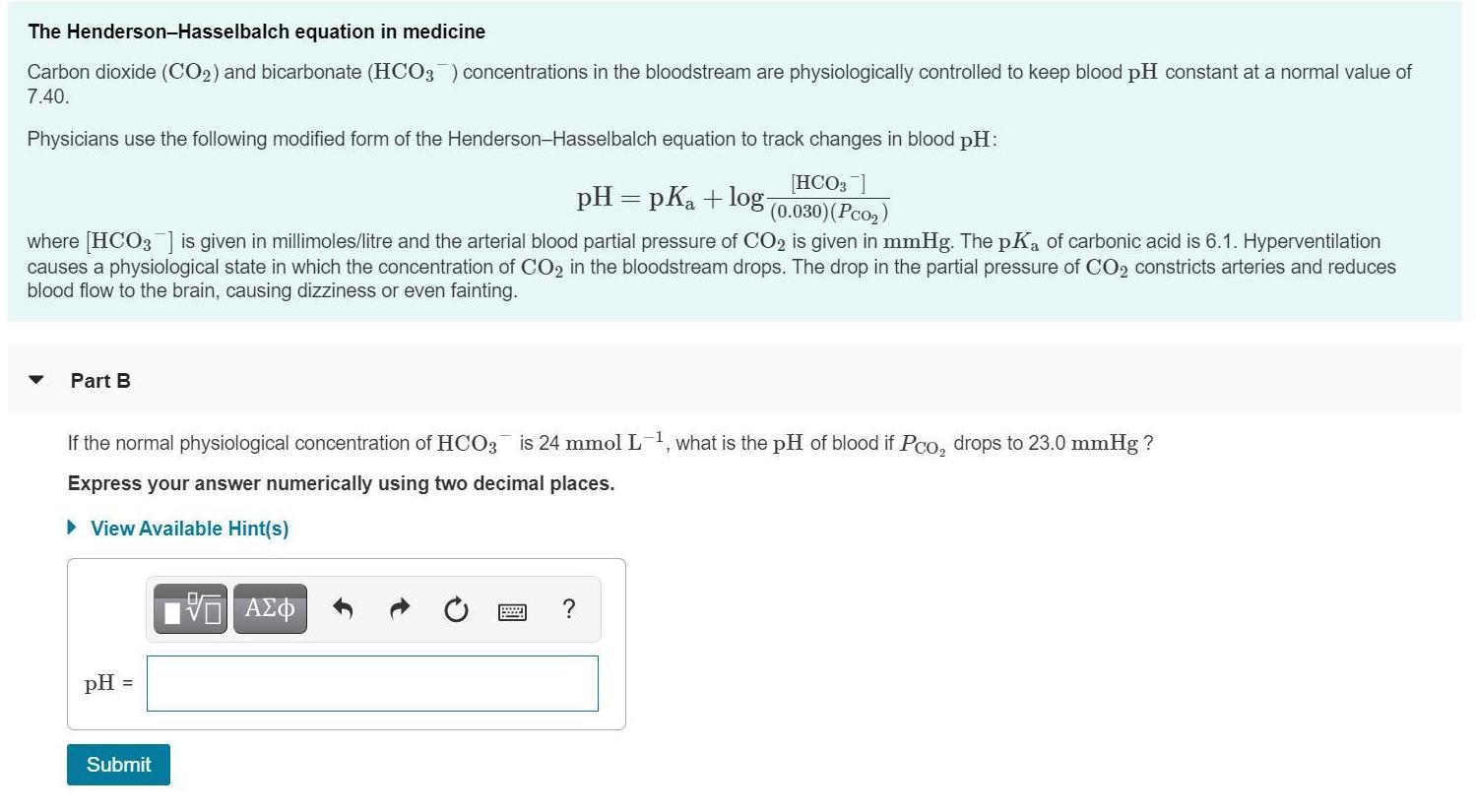
The Henderson-Hasselbalch equation in medicine Carbon dioxide (CO2) and bicarbonate (HCO3) concentrations in the bloodstream are physiologically controlled to keep blood pH constant at a normal value of 7.40. Physicians use the following modified form of the Henderson-Hasselbalch equation to track changes in blood pH: [HCO3 ] pH = pK + log- (0.030) (Pco) where [HCO3] is given in millimoles/litre and the arterial blood partial pressure of CO2 is given in mmHg. The pKa of carbonic acid is 6.1. Hyperventilation causes a physiological state in which the concentration of CO2 in the bloodstream drops. The drop in the partial pressure of CO2 constricts arteries and reduces blood flow to the brain, causing dizziness or even fainting. Part B If the normal physiological concentration of HCO3 is 24 mmol L-1, what is the pH of blood if Pco drops to 23.0 mmHg ? Express your answer numerically using two decimal places. View Available Hint(s) VE ? pH = Submit
Step by Step Solution
★★★★★
3.32 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
pH pKa log...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


