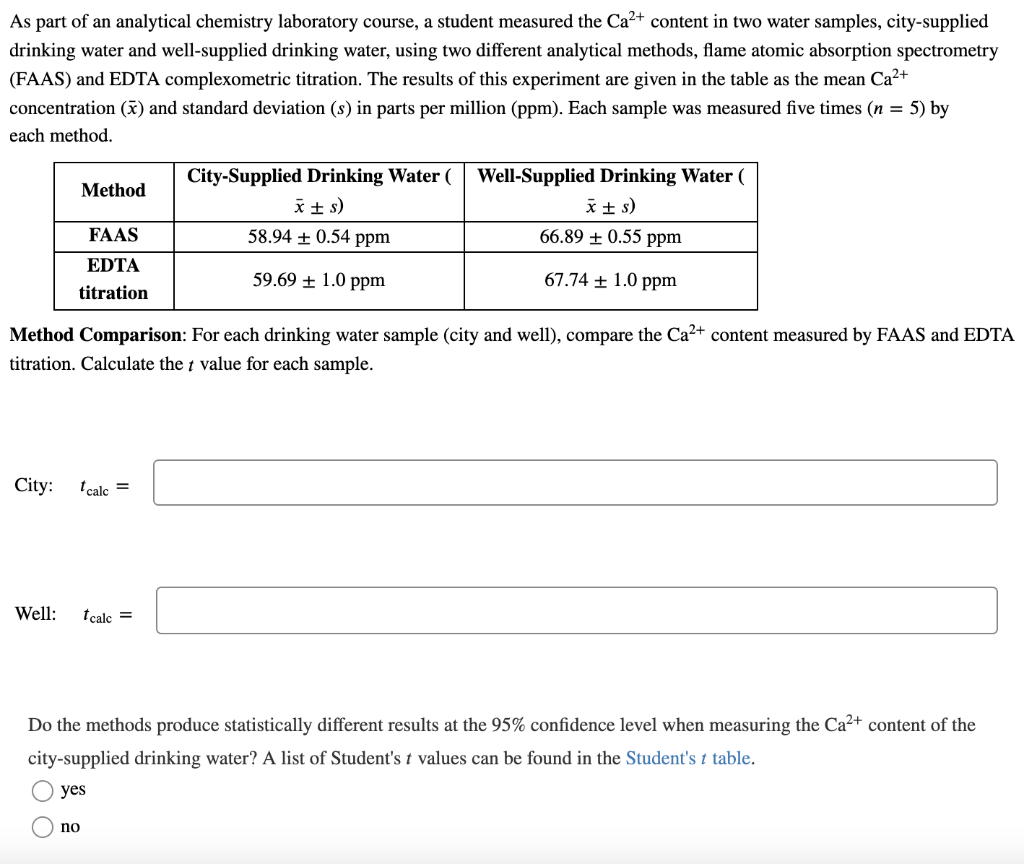
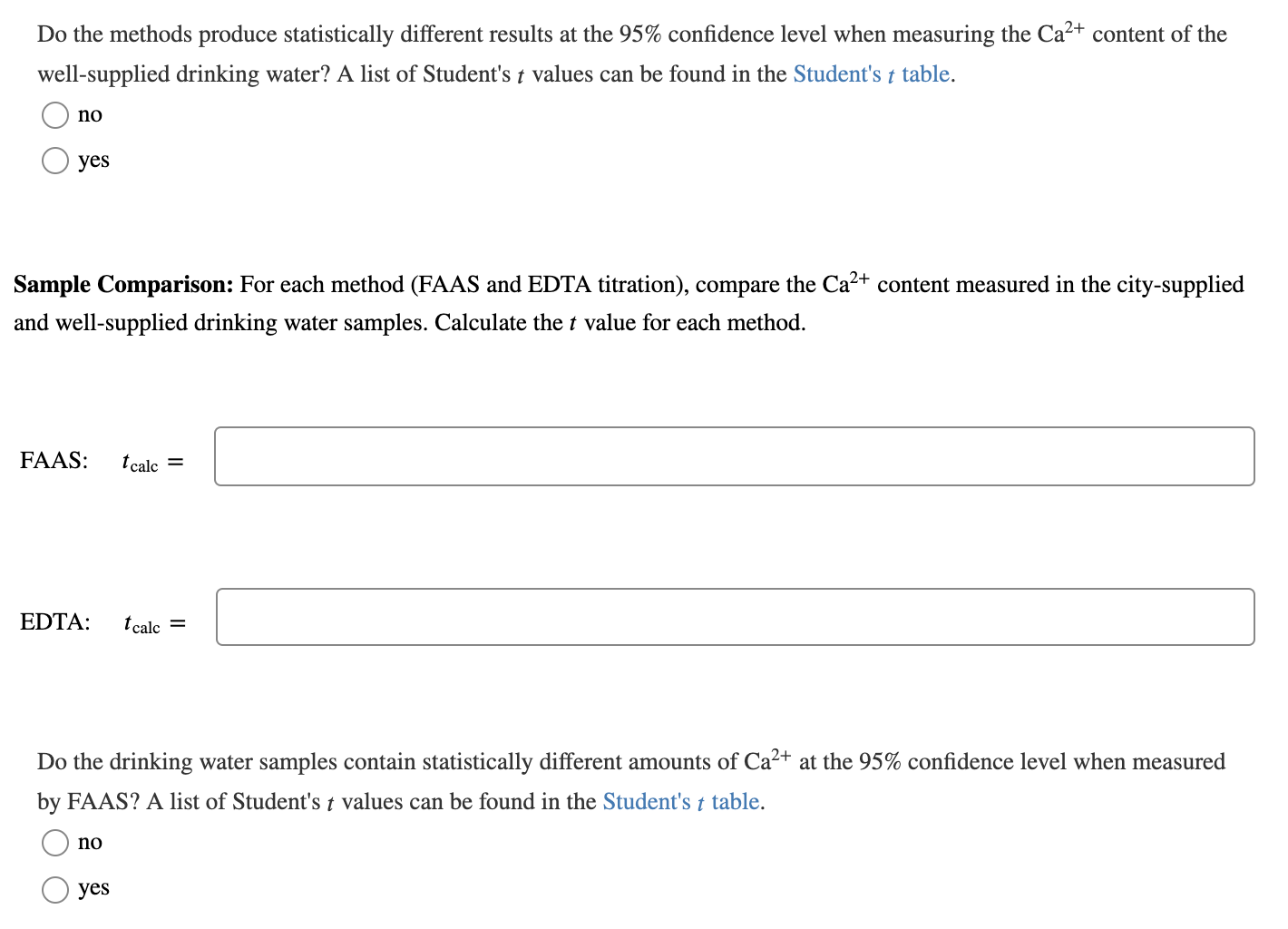

As part of an analytical chemistry laboratory course, a student measured the Ca2+ content in two water samples, city-supplied drinking water and well-supplied drinking water, using two different analytical methods, flame atomic absorption spectrometry (FAAS) and EDTA complexometric titration. The results of this experiment are given in the table as the mean Ca2+ concentration (x) and standard deviation (s) in parts per million (ppm). Each sample was measured five times (n = 5) by each method. Method City-Supplied Drinking Water ( x +) 58.94 + 0.54 ppm Well-Supplied Drinking Water ( *+s) 66.89 + 0.55 ppm FAAS EDTA titration 59.69 + 1.0 ppm 67.74 + 1.0 ppm Method Comparison: For each drinking water sample (city and well), compare the Ca2+ content measured by FAAS and EDTA titration. Calculate the t value for each sample. City: I calc = Well: I calc = Do the methods produce statistically different results at the 95% confidence level when measuring the Ca2+ content of the city-supplied drinking water? A list of Student's t values can be found in the Student's t table. yes no Do the methods produce statistically different results at the 95% confidence level when measuring the Ca2+ content of the well-supplied drinking water? A list of Student's t values can be found in the Student's t table. no yes Sample Comparison: For each method (FAAS and EDTA titration), compare the Ca2+ content measured in the city-supplied and well-supplied drinking water samples. Calculate the t value for each method. FAAS: t calc = EDTA: t calc = Do the drinking water samples contain statistically different amounts of Ca2+ at the 95% confidence level when measured by FAAS? A list of Student's t values can be found in the Student's t table. t no yes Do the drinking water samples contain statistically different amounts of Ca2+ at the 95% confidence level when measured by EDTA titration? A list of Student's t values can be found in the Student's t table. no yes As part of an analytical chemistry laboratory course, a student measured the Ca2+ content in two water samples, city-supplied drinking water and well-supplied drinking water, using two different analytical methods, flame atomic absorption spectrometry (FAAS) and EDTA complexometric titration. The results of this experiment are given in the table as the mean Ca2+ concentration (x) and standard deviation (s) in parts per million (ppm). Each sample was measured five times (n = 5) by each method. Method City-Supplied Drinking Water ( x +) 58.94 + 0.54 ppm Well-Supplied Drinking Water ( *+s) 66.89 + 0.55 ppm FAAS EDTA titration 59.69 + 1.0 ppm 67.74 + 1.0 ppm Method Comparison: For each drinking water sample (city and well), compare the Ca2+ content measured by FAAS and EDTA titration. Calculate the t value for each sample. City: I calc = Well: I calc = Do the methods produce statistically different results at the 95% confidence level when measuring the Ca2+ content of the city-supplied drinking water? A list of Student's t values can be found in the Student's t table. yes no Do the methods produce statistically different results at the 95% confidence level when measuring the Ca2+ content of the well-supplied drinking water? A list of Student's t values can be found in the Student's t table. no yes Sample Comparison: For each method (FAAS and EDTA titration), compare the Ca2+ content measured in the city-supplied and well-supplied drinking water samples. Calculate the t value for each method. FAAS: t calc = EDTA: t calc = Do the drinking water samples contain statistically different amounts of Ca2+ at the 95% confidence level when measured by FAAS? A list of Student's t values can be found in the Student's t table. t no yes Do the drinking water samples contain statistically different amounts of Ca2+ at the 95% confidence level when measured by EDTA titration? A list of Student's t values can be found in the Student's t table. no yes









