Answered step by step
Verified Expert Solution
Question
1 Approved Answer
As shown in the diagram, a water molecule at the origin is a permanent electric dipole: it is charged positively on the left where

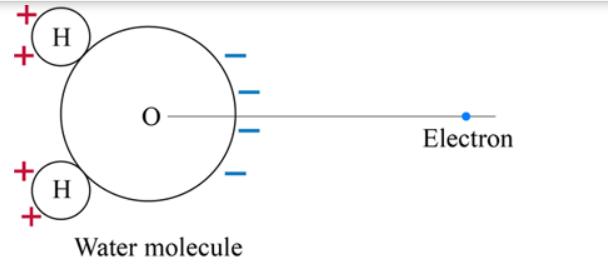
As shown in the diagram, a water molecule at the origin is a "permanent electric dipole": it is charged positively on the left where the two hydrogen atoms are, and is charged negatively on the right where the oxygen atom is. This combination of charges exerts a force to the right on an electron located to the right of the water molecule. The force is to the right because the negatively charged region of the water molecule is closer to the electron than is the positively charged region. The force exerted on the electron is not proportional to 1/x because the effects of the positive and negative regions of the water molecule nearly cancel each other out. Rather, the force on the electron is proportional to 1/x and is equal to 1.12 10-19 e/x N, where the magnitude of the electron charge is e = 1.6 10-19 C. The electron is initially at rest at x; = 16 10-10 m. It accelerates to the right, along the x axis. At the moment that it passes x = 25 10-10 m, how much work has been done on the electron? (Because the water molecule's mass is much greater than the electron's mass, the water molecule hardly moves during this time.) work= + H H Water molecule Electron
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


