Answered step by step
Verified Expert Solution
Question
1 Approved Answer
As shown in the figure below, you have a system including a Steel vessel with mass Myessel = 0.276 kg. The vessel contains water
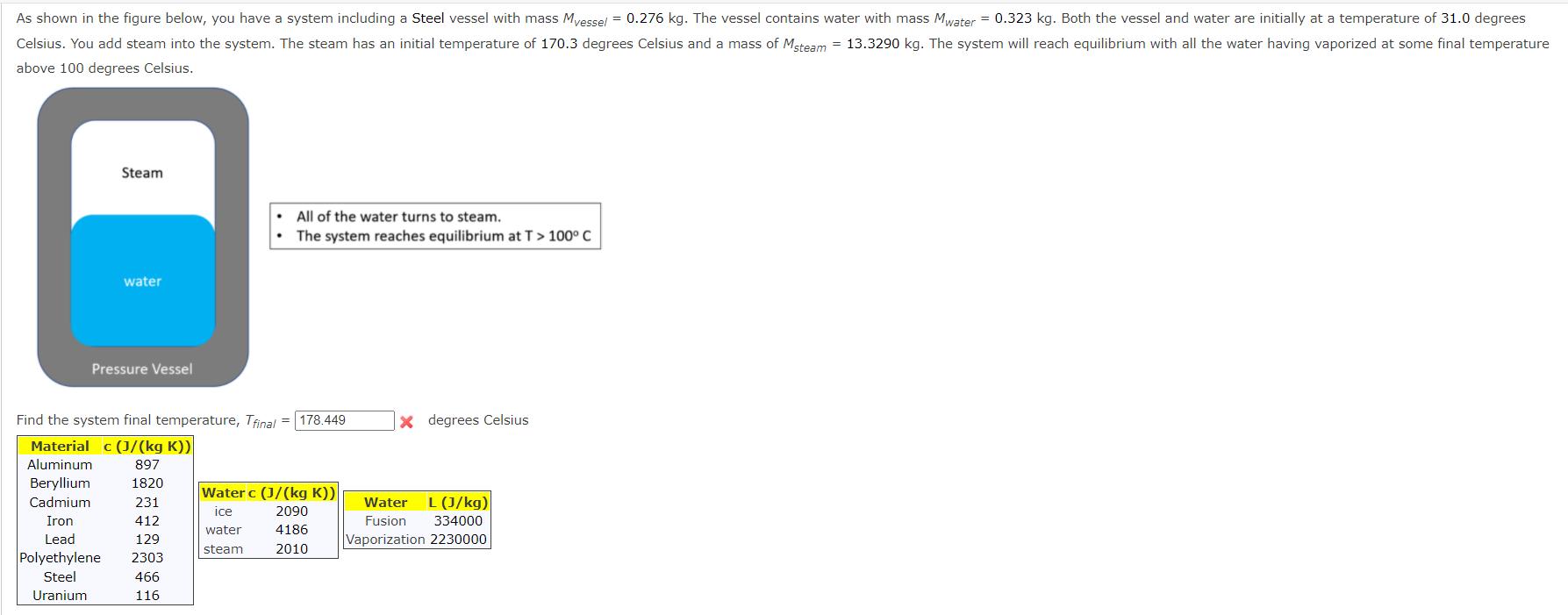
As shown in the figure below, you have a system including a Steel vessel with mass Myessel = 0.276 kg. The vessel contains water with mass Mwater = 0.323 kg. Both the vessel and water are initially at a temperature of 31.0 degrees Celsius. You add steam into the system. The steam has an initial temperature of 170.3 degrees Celsius and a mass of Msteam = 13.3290 kg. The system will reach equilibrium with all the water having vaporized at some final temperature above 100 degrees Celsius. Steam water Pressure Vessel All of the water turns to steam. The system reaches equilibrium at T > 100 C Find the system final temperature, Tfinal= 178.449 Material c (J/(kg K)) Aluminum 897 Beryllium Cadmium Iron Lead 1820 231 412 129 Polyethylene 2303 Steel 466 Uranium 116 Water c (J/(kg K)) ice 2090 water 4186 steam 2010 X degrees Celsius Water L (J/kg) Fusion Vaporization 2230000 334000
Step by Step Solution
★★★★★
3.37 Rating (166 Votes )
There are 3 Steps involved in it
Step: 1
principle of method of mixtures by hot body heat gained by cold body Acc to he...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


