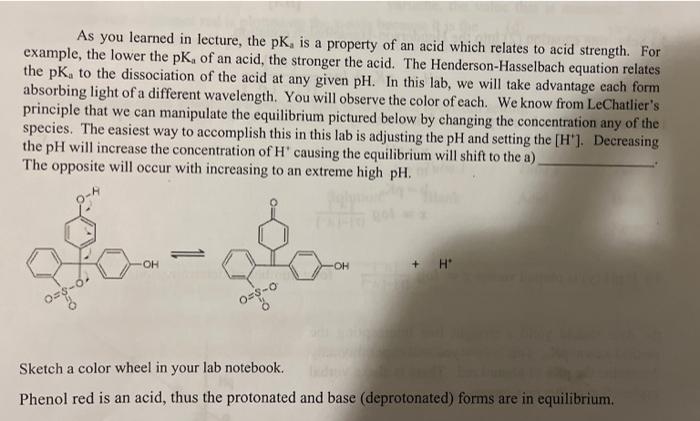
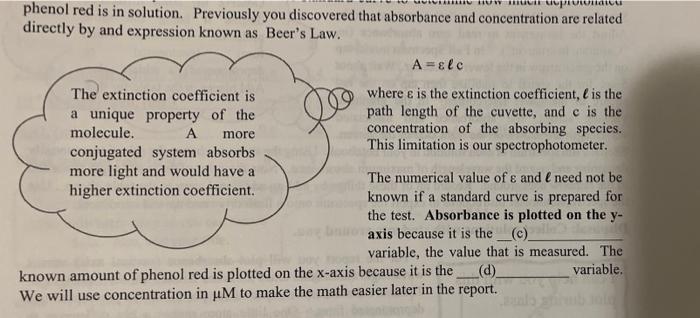
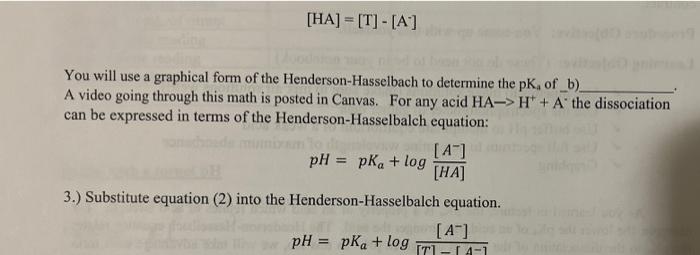As you learned in lecture, the pK, is a property of an acid which relates to acid strength. For example, the lower the pK, of an acid, the stronger the acid. The Henderson-Hasselbach equation relates the pK, to the dissociation of the acid at any given pH. In this lab, we will take advantage each form absorbing light of a different wavelength. You will observe the color of each. We know from LeChatlier's principle that we can manipulate the equilibrium pictured below by changing the concentration any of the species. The easiest way to accomplish this in this lab is adjusting the pH and setting the [H"]. Decreasing the pH will increase the concentration of H' causing the equilibrium will shift to the a) The opposite will occur with increasing to an extreme high pH. OH OH + H 08-6. 02-0 Sketch a color wheel in your lab notebook. Phenol red is an acid, thus the protonated and base (deprotonated) forms are in equilibrium. [HA] = [T] - [A] You will use a graphical form of the Henderson-Hasselbach to determine the pK, of_b) A video going through this math is posted in Canvas. For any acid HA-> H+ + A the dissociation can be expressed in terms of the Henderson-Hasselbalch equation: [A] pH pka + log [HA] 3.) Substitute equation (2) into the Henderson-Hasselbalch equation. [A] pH = pka + log -14-1 = HUN MUP phenol red is in solution. Previously you discovered that absorbance and concentration are related directly by and expression known as Beer's Law. A=&lc The extinction coefficient is where e is the extinction coefficient, l is the a unique property of the path length of the cuvette, and c is the molecule. A more concentration of the absorbing species. This limitation is our spectrophotometer. conjugated system absorbs more light and would have a The numerical value of e and I need not be higher extinction coefficient known if a standard curve is prepared for the test. Absorbance is plotted on the y- axis because it is the _(c) variable, the value that is measured. The known amount of phenol red is plotted on the x-axis because it is the (d) variable. We will use concentration in uM to make the math easier later in the report