Answered step by step
Verified Expert Solution
Question
1 Approved Answer
ASAP ASAP!!!! = Problem 1 The West Virginia Water Research Institute reported the following water quality measurements for the Monongahela River monitoring station at Mile
ASAP ASAP!!!! 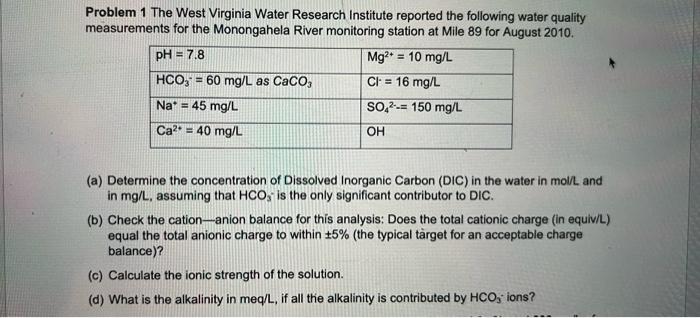
= Problem 1 The West Virginia Water Research Institute reported the following water quality measurements for the Monongahela River monitoring station at Mile 89 for August 2010 pH = 7.8 Mg2+ = 10 mg/L HCO," = 60 mg/L as CaCO3 Cl = 16 mg/L Na* = 45 mg/L SO2--= 150 mg/l Ca2+ = 40 mg/l OH (a) Determine the concentration of Dissolved Inorganic Carbon (DIC) in the water in mol/L and in mg/L, assuming that HCO, is the only significant contributor to DIC. (b) Check the cation-anion balance for this analysis: Does the total cationic charge (in equiv/L) equal the total anionic charge to within 5% (the typical target for an acceptable charge balance)? (c) Calculate the ionic strength of the solution. (d) What is the alkalinity in meq/L, if all the alkalinity is contributed by HCO3-ions 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


