Answered step by step
Verified Expert Solution
Question
1 Approved Answer
ASAP please The goal of this assignment is to give you more practice identifying if a reaction will go through an SN1, SN2, E1, or
ASAP please 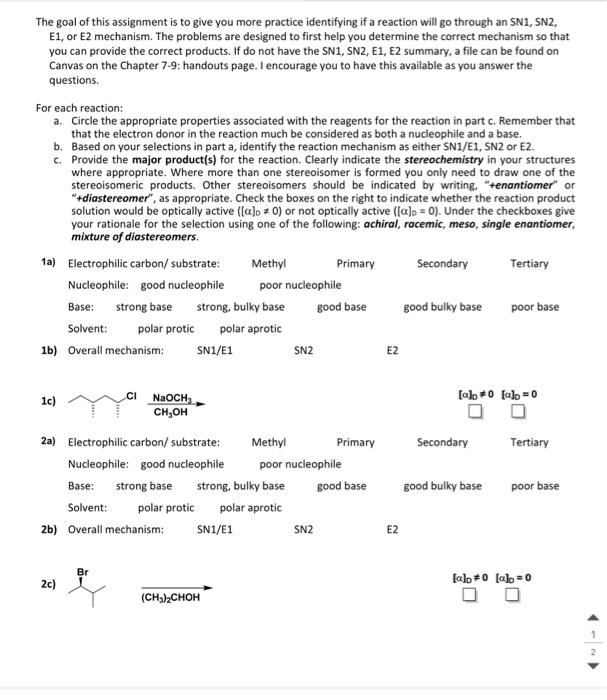
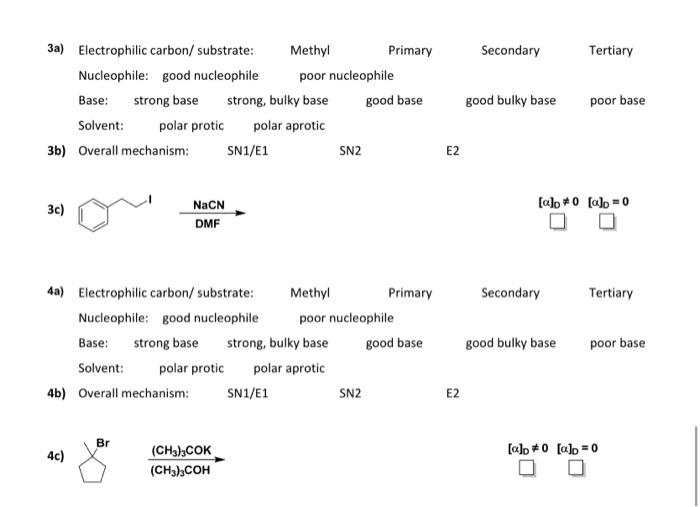
The goal of this assignment is to give you more practice identifying if a reaction will go through an SN1, SN2, E1, or E2 mechanism. The problems are designed to first help you determine the correct mechanism so that you can provide the correct products. If do not have the SN1, SN2, E1, E2 summary, a file can be found on Canvas on the Chapter 7-9: handouts page. I encourage you to have this available as you answer the questions. For each reaction: a. Circle the appropriate properties associated with the reagents for the reaction in part c. Remember that that the electron donor in the reaction much be considered as both a nucleophile and a base. b. Based on your selections in part a, identify the reaction mechanism as either SN1/E1, SN2 or E2. c. Provide the major product(s) for the reaction. Clearly indicate the stereochemistry in your structures where appropriate. Where more than one stereoisomer is formed you only need to draw one of the stereoisomeric products. Other stereoisomers should be indicated by writing, "+enantiomer" or "+diastereomer", as appropriate. Check the boxes on the right to indicate whether the reaction product solution would be optically active ([]0=0) or not optically active ([]0=0). Under the checkboxes give your rationale for the selection using one of the following: achiral, racemic, meso, single enantiomer, mixture of diastereomers. 1a) Electrophilic carbon/substrate: Methyl Primary Secondary Tertiary Nucleophile: good nucleophile poor nucleophile Base: strong base strong, bulky base good base good bulky base poor base Solvent: polarprotic polar aprotic 1b) Overall mechanism: SN1/E1 SN2 E2 1c) 2a) Electrophilic carbon/substrate: Methyl Primary Secondary Tertiary Nucleophile: good nucleophile poor nucleophile Base: strong base strong, bulky base good base good bulky base poor base Solvent: polar protic polaraprotic 2b) Overall mechanism: SN1/E1 SN2 E2 2c) (CH3)2CHOH []D=0[]D=0 3a) Electrophilic carbon/substrate: Methyl Primary Secondary Tertiary Nucleophile: good nucleophile poor nucleophile Base: strong base strong, bulky base good base good bulky base poor Solvent: polar protic polar aprotic 3b) Overall mechanism: SN1/E1 SN2 E2 3c) DMFNaCN []D=0[]D=0 4a) Electrophilic carbon/substrate: Methyl Primary Secondary Tertiary Nucleophile: good nucleophile poor nucleophile Base: strong base strong, bulky base good base good bulky base base Solvent: polar protic polar aprotic 4b) Overall mechanism: SN1/E1 SN2 E2 4c) (CH3)3COH(CH3)3COK []D=0[]D=0 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


