Answered step by step
Verified Expert Solution
Question
1 Approved Answer
ASSICNMENT 2 ( 1 5 % ) s cizas LABORATORY QUALTY MANAGEMENT DAN VALDATION OPTION 2 Deneminaton of benzoic acid in soya sauce by HPLC
ASSICNMENT
scizas
LABORATORY QUALTY MANAGEMENT DAN VALDATION
OPTION
Deneminaton of benzoic acid in soya sauce by HPLC
Procodure:
Weigh sampie into graduated tlask.
Add acetic acid:
Add intemal standard methy parsten
Add acetonitrile to the mark, titer sample with menterane titer
Calibrate HPLC with standard at levels between pom berzoic ackd standard
Inject of each standard
Inject uL of sample solution
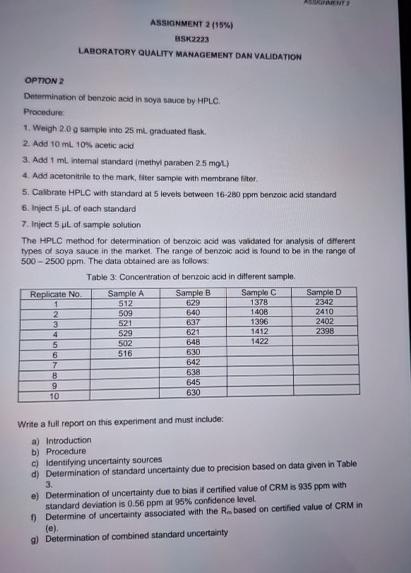
The HPLC method for determination of berzoic acid was vabdated for analysis of differert types of soya sauce in the market. The range of benzoic acid is found to be in the range of ppm The data obeained are as follows.
Table : Concerveration of benzoic acid in different sample.
tableReplicate NoSample ASample BSample CSample D
Write a lull report on this experiment and must include:
a Introduction
b Procedure
c Identifying uncertainty sources
d Determination of standard uncertainly due to precision based on data givon in Table
e Determination of uncertainty due to bias if certified value of CRM is ppm with standard deviation is ppm at confidence lovel.
Detenmine of uncertainty associated with the Ris based on certifed value of CRM in e
g Determination of corrbined standard uncertainty
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


