Question
At 1 atm, how much energy is required to heat 89.0 g of HO(s) at-16.0 C to HO(g) at 119.0 C? Helpful constants can
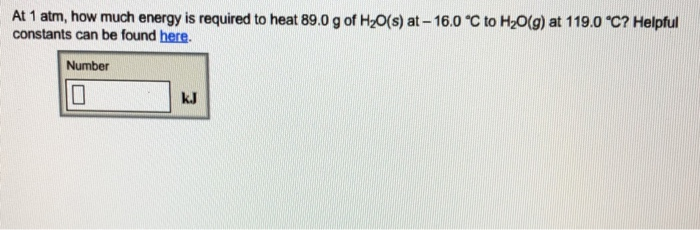
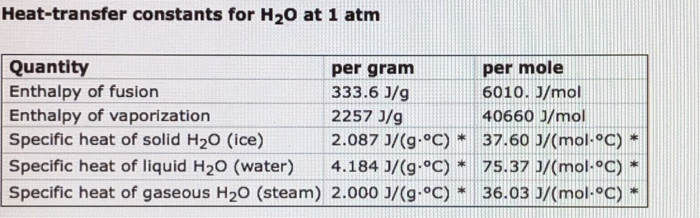
At 1 atm, how much energy is required to heat 89.0 g of HO(s) at-16.0 C to HO(g) at 119.0 C? Helpful constants can be found here. Number kJ Heat-transfer constants for H0 at 1 atm Quantity Enthalpy of fusion Enthalpy of vaporization Specific heat of solid HO (ice) 2.087 J/(g-C) * Specific heat of liquid HO (water) 4.184 J/(g-C)* Specific heat of gaseous HO (steam) 2.000 J/(g-C) * per gram 333.6 J/g 2257 J/g per mole 6010. J/mol 40660 J/mol 37.60 J/(mol-C) 75.37 0/(mol-C) 36.03 J/(mol-C)
Step by Step Solution
3.50 Rating (170 Votes )
There are 3 Steps involved in it
Step: 1
Answer Energy required to ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics
Authors: Alan Giambattista, Betty Richardson, Robert Richardson
2nd edition
77339681, 978-0077339685
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



