Question
at 17) Normal boiling point of water is 100 C. What is the vapor pressure of an aqueous solution at 100 C containing 15.1%
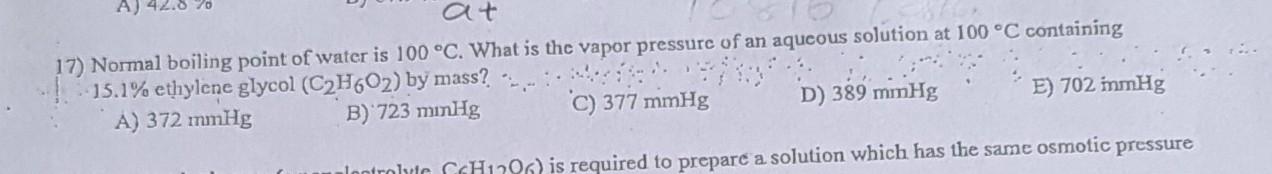
at 17) Normal boiling point of water is 100 C. What is the vapor pressure of an aqueous solution at 100 C containing 15.1% ethylene glycol (C2H602) by mass? A) 372 mmHg B) 723 minHg C) 377 mmHg D) 389 mmHg lontrolte CH1706) is required to prepare a solution which has the same osmotic pressure E) 702 immHg
Step by Step Solution
3.54 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
SOLUTION To solve this problem we need to use the formula for vapor pressure of an aqueous solution which is P P0 m100 where P is the vapor pressure o...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



