Question
At a certain temperature the rate of this reaction is first order in H,CO, with a rate constant of 1.58 s : H,Co, (ag)
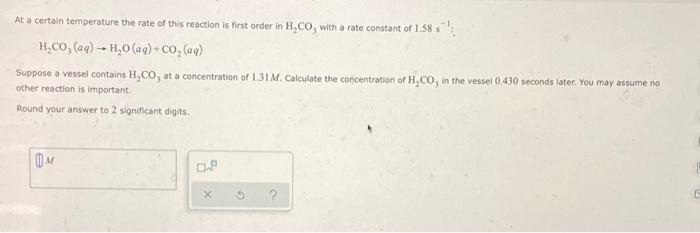
At a certain temperature the rate of this reaction is first order in H,CO, with a rate constant of 1.58 s : H,Co, (ag) -- H,0 (aq) +co, (aq) Suppose a vessel contains H,CO, at a concentration of 1.31M. Calculate the concentration of H,CO, in the vessel 0.430 seconds later. You may assume no other reaction is important. Round your answer to 2 significant digits.
Step by Step Solution
3.45 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
At a specific temperature the rate of change of concentration of t...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Joseph M. Hornback
2nd Edition
9781133384847, 9780199270293, 534389511, 1133384846, 978-0534389512
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



