Answered step by step
Verified Expert Solution
Question
1 Approved Answer
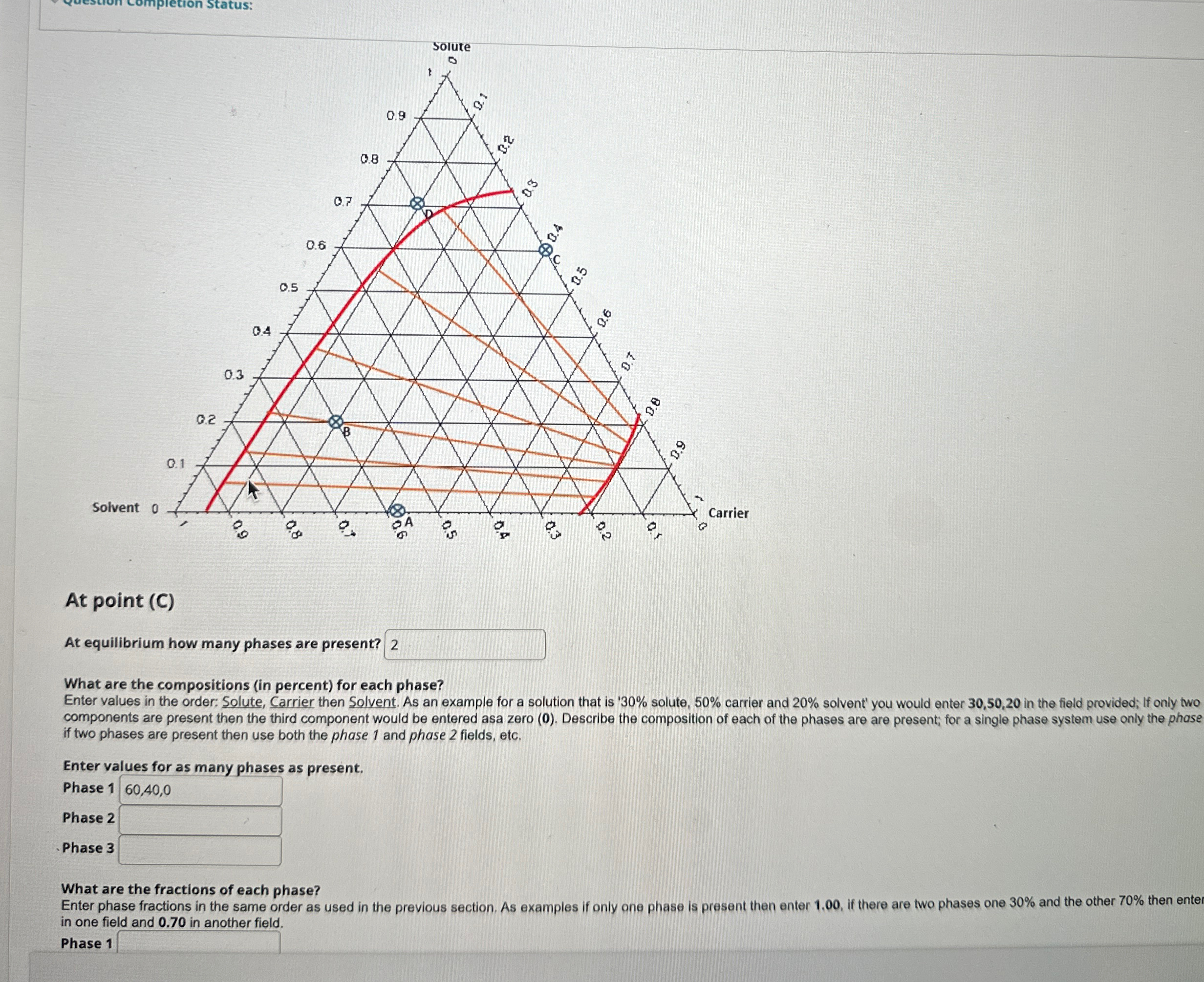
At point ( C ) At equilibrium how many phases are present? What are the compositions ( in percent ) for each phase? Enter values
At point C
At equilibrium how many phases are present?
What are the compositions in percent for each phase?
Enter values in the order: Solute, Carrier then Solvent. As an example for a solution that is solute, carrier and solvent' you would enter in the field provided; If only two components are present then the third component would be entered asa zero Describe the composition of each of the phases are are present; for a single phase system use only the phase if two phases are present then use both the phase and phase fields, etc.
Enter values for as many phases as present.
Phase
Phase
Phase
What are the fractions of each phase?
Enter phase fractions in the same order as used in the previous section. As examples if only one phase is present then enter if there are two phases one and the other then enter in one field and in another field.
Phase
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


