Question
At T =300K, 1bar of 00 in a 1m box (lengths a= ay = a, = 1m) can be considered as an ideal gas.
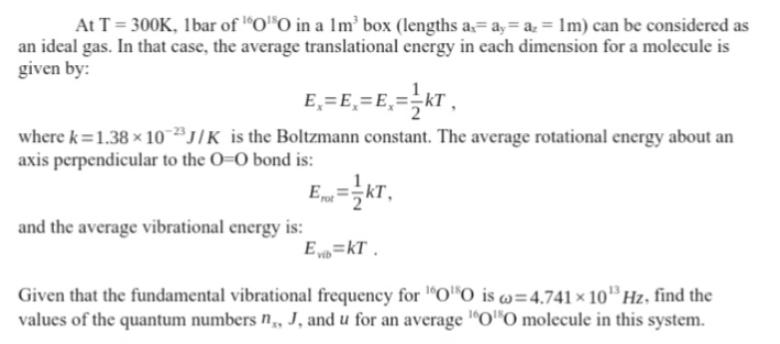
At T =300K, 1bar of "00 in a 1m box (lengths a= ay = a, = 1m) can be considered as an ideal gas. In that case, the average translational energy in each dimension for a molecule is given by: E=E=E= KT where k=1.38 x 10 J/K is the Boltzmann constant. The average rotational energy about an axis perpendicular to the O-O bond is: Ert-kT, and the average vibrational energy is: Evib kT. Given that the fundamental vibrational frequency for OO is w=4.741 10 Hz, find the values of the quantum numbers n., J, and u for an average 0"O molecule in this system.
Step by Step Solution
3.51 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
the average translational energy for a molecule in each dimension is give...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Modern Classical Physics Optics Fluids Plasmas Elasticity Relativity And Statistical Physics
Authors: Kip S. Thorne, Roger D. Blandford
1st Edition
0691159025, 978-0691159027
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



