Answered step by step
Verified Expert Solution
Question
1 Approved Answer
At-12.7 C the concentration equilibrium constant K =5.3 x 10 for a certain reaction. Here are some facts about the reaction: The constant pressure
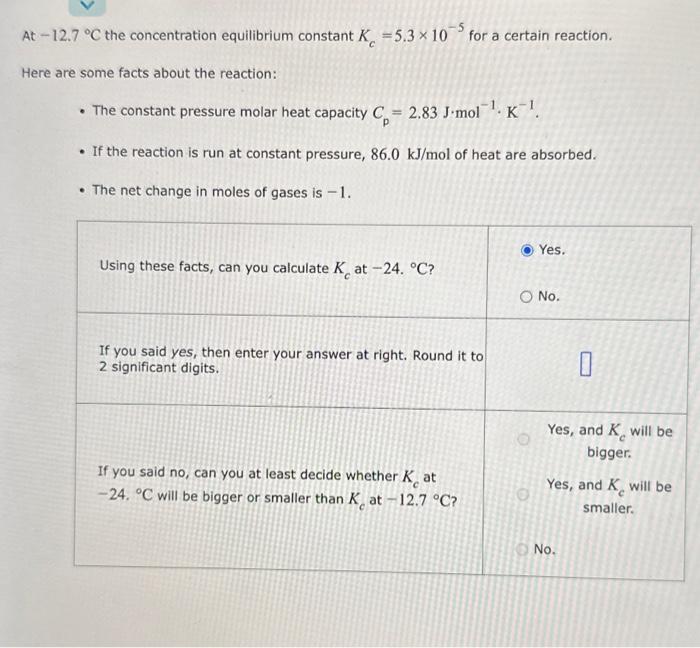
At-12.7 C the concentration equilibrium constant K =5.3 x 10 for a certain reaction. Here are some facts about the reaction: The constant pressure molar heat capacity C= 2.83 J-mol. K. If the reaction is run at constant pressure, 86.0 kJ/mol of heat are absorbed. The net change in moles of gases is -1. Using these facts, can you calculate K at -24. C? Yes. O No. If you said yes, then enter your answer at right. Round it to 2 significant digits. 0 If you said no, can you at least decide whether Kat -24. C will be bigger or smaller than K at -12.7 C? Yes, and K will be bigger. Yes, and K will be smaller. No.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


