Question
(a)The diagram below represents part of the structure of a sodium chloride crystal. The position of one of the sodium ions in the crystal is
(a)The diagram below represents part of the structure of a sodium chloride crystal. The position of one of the sodium ions in the crystal is shown as
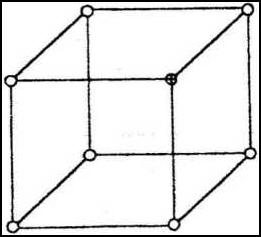
(i) On the diagram, mark the position of the other three sodium ions(2 marks)
(ii) The melting and boiling points of sodium chloride are 801°C and 1413°C respectively. Explain why sodium chloride does not conduct electricity at 25°C, but does so at temperatures between 801° C and 1413°C.
(b) Give a reason why ammonia gas is highly soluble in water.
(c) The structure of an ammonia ion is shown below: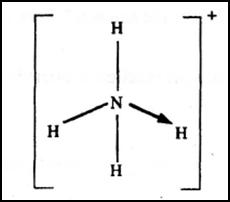
Name the type of bond represented in the diagram by N→ H
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


