Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Average seawater has the following chemical composition in ppm (or mg/L): In this problem, you cannot assume that a = m, so you will have
Average seawater has the following chemical composition in ppm (or mg/L):
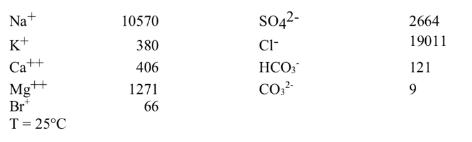
In this problem, you cannot assume that a = m, so you will have to calculate activities for some species using the Truesdell-Jones equation. Answer the following and state your assumptions:
a) What are these concentrations in moles/kg?
b) Do the charges balance (total + = total -)?
c) Would NaCl precipitate from this water i.e. calculate the state of halite saturation (SI index), where:
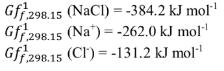
Step by Step Solution
★★★★★
3.44 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


