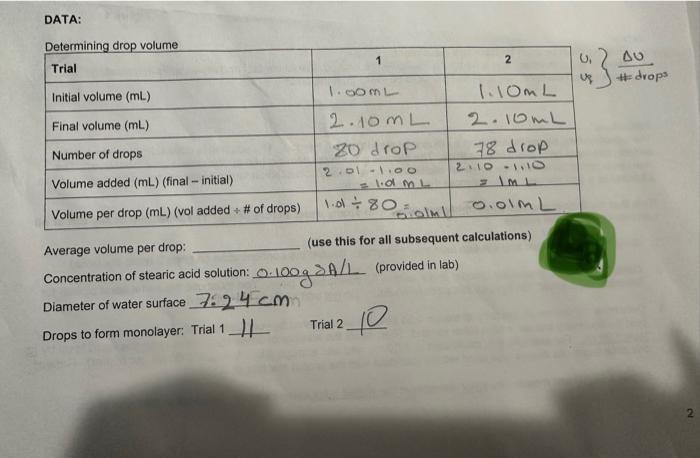
Average volume per drop: (use this for all subsequent calculations) Concentration of stearic acid solution: 0.100gA/L (provided in lab) Diameter of water surface 7.24cm Drops to form monolayer: Trial 1 Trial 2 CALCULATIONS Remember: work ALONE on lab reports - and always show your work You must use dimensional analysis for questions #2,#6, \#\#8, and #91 1. Calculate the surface area of the monolayer (= water surface) (in cm2 ) using the calipers measurement. A=r2 A=c2=(7.24)2=164.67cm2 2. Calculate the grams of stearic acid molecules used to form the monolayer in each trial via dimensional analysis: start with A of drops to form monolayer x average volume per drop ( mLd drop). You will be using the calculation you entered below the table on p. 2, and you will need to use the concentration of the stearic acid soluiion. Do this for each trial: onolayer \#F 11 3. Use the density of pure stearic acid (0.941g/mL ) to calculate the volume of stearic acid in an average monolayer (use the average mass from calculation \#\#2). 4. Use the fact that volume = area thickness to calculate the thickness (t) of the monolayer in cm: 5. If we assume that each molecule of stearic acid is six times longer than its diameter, and that it has a cylindrical shape, the volume of each molecule can be expressed by the following equation, where t is the monolayer thickness: V=144t3 Use this equation and your result for calculation #4 to find the volume of a single molecule in mL : 6. Use the density of stearic acid (g/mL), the volume of a molecule ( mL/molecule), and the fact that 1 mole of stearic acid =284g to calculate the number of molecules per mole by dimensional analysis. Since you are trying to calculate Avogadro's number, you cannot use 6.0221023 to solve this problem! 1mole284gx 7. Comment on the accuracy of your result for \#6: how does it compare to the expected value for Avogadro's number? A reasonable answer for 46 in this experiment is within a power of 10 of the expected result (use ONE complete sentence; refer to your result and the expected result clearly): 8. Using your molecular volume (mLimolecule, H5): calculate the volume (in L) of 500.0 moles of this molecule. Use dimensional analysis (and use the expected value for Avogadro's number): 9. Suppose a different fatty acid molecule were used, with a molecular volume of 4.481022mL/molecule, and a molar mass of 235g/mol. Use these data and the expected value for Avogadro's number to calculate its density ( 9mL ) by dimensional analysis. Hint try starting with 235g over 1 mole