Question
Avogadro's number, No, of monatomic ideal gas particles undergoes this cycle: starting from Po, To, it contracts adiabatically to 2Po, then it expands isothermally,
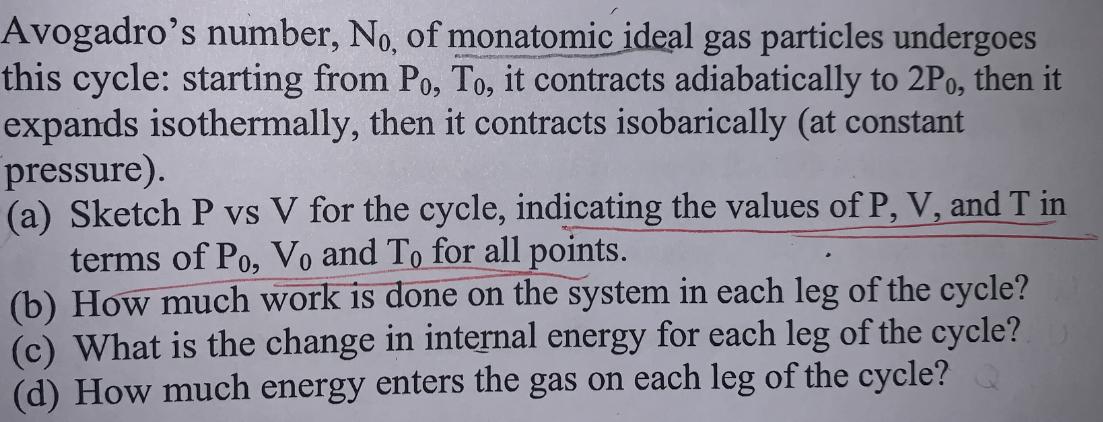
Avogadro's number, No, of monatomic ideal gas particles undergoes this cycle: starting from Po, To, it contracts adiabatically to 2Po, then it expands isothermally, then it contracts isobarically (at constant pressure). (a) Sketch P vs V for the cycle, indicating the values of P, V, and T in terms of Po, Vo and To for all points. (b) How much work is done on the system in each leg of the cycle? (c) What is the change in internal energy for each leg of the cycle? (d) How much energy enters the gas on each leg of the cycle?
Step by Step Solution
3.44 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
Solution for the ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Ethics for Scientists and Engineers
Authors: Edmund G. Seebauer, Robert L. Barry
1st Edition
9780195698480, 195134885, 195698487, 978-0195134889
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



