Answered step by step
Verified Expert Solution
Question
1 Approved Answer
b 4 Question (6 points) a Seepage 1026 Many organic syntheses exploit the presence of a particular functional group that is not present in the
b 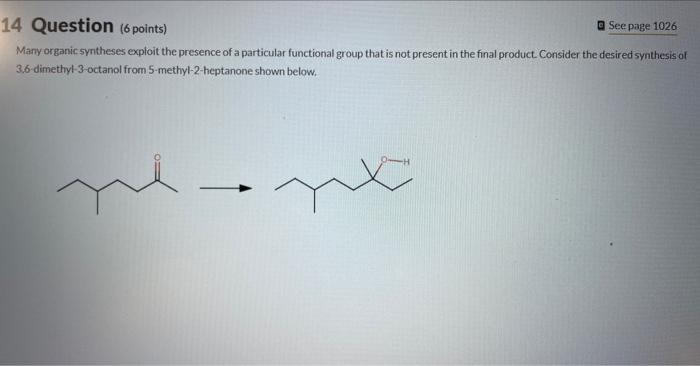
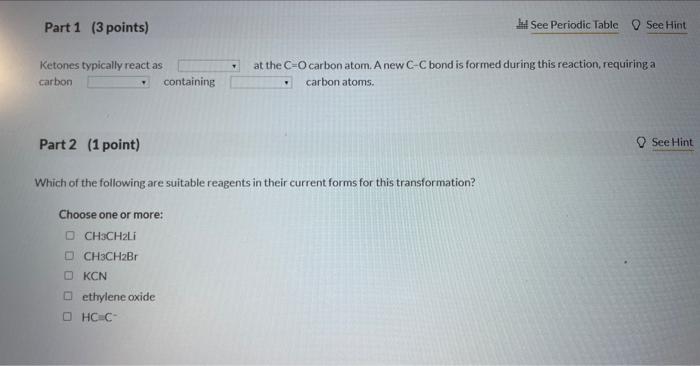
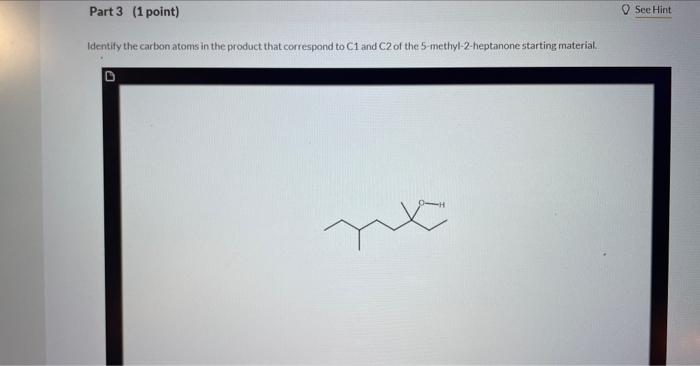
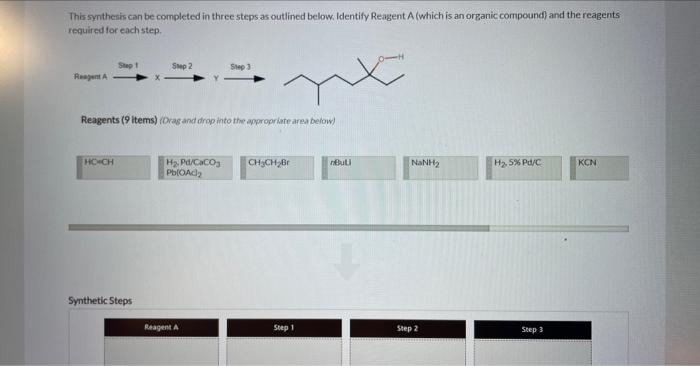
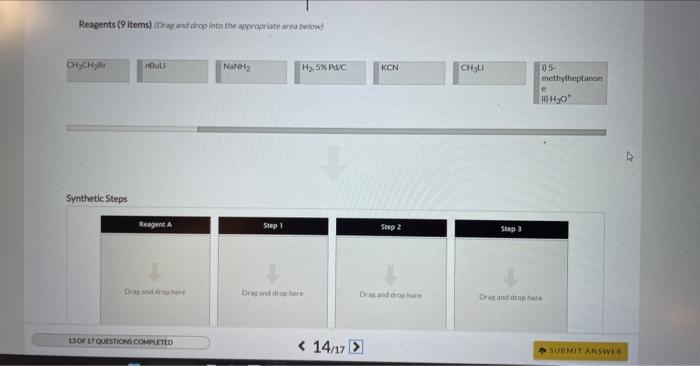
4 Question (6 points) a Seepage 1026 Many organic syntheses exploit the presence of a particular functional group that is not present in the final product. Consider the desired synthesis of 3.6 dimethyl-3-octanol from 5-methyl-2-heptanone shown below. Ketones typically react as at the C=O carbon atom. A new CC bond is formed during this reaction, requiring a carbon containing carbon atoms. Part 2 (1 point) Which of the following are suitable reagents in their current forms for this transformation? Choose one or more: CH3CH2Li CH3CH2Br KCN ethylene oxide HCC Identify the carbon atoms in the product that correspond to C1 and C2 of the 5 -methyl-2-heptanone starting material. This synthenis can be completed in three steps as outlined below. Identify Reagent A (which is an organic compound) and the reagents required for each step. Reagents ( 9 items) (Drag and drop into the eppropriate area below) Reagents (9 items) (Dray and drop into the apponoporiute area below) 




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


