Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(b) A biotechnologist attempted to bioengineer parasites to produce enzyme systems to convert L-amino acids to D-amino acids in an effort to produce these unnatural
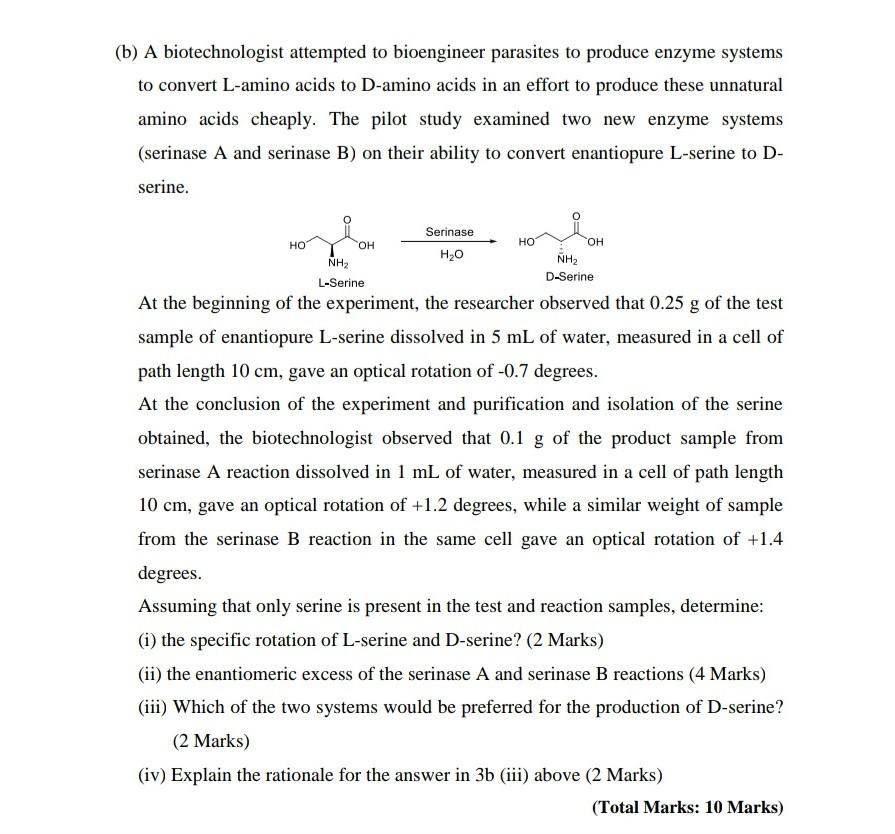
(b) A biotechnologist attempted to bioengineer parasites to produce enzyme systems to convert L-amino acids to D-amino acids in an effort to produce these unnatural amino acids cheaply. The pilot study examined two new enzyme systems (serinase A and serinase B) on their ability to convert enantiopure L-serine to Dserine. At the beginning of the experiment, the researcher observed that 0.25g of the test sample of enantiopure L-serine dissolved in 5mL of water, measured in a cell of path length 10cm, gave an optical rotation of -0.7 degrees. At the conclusion of the experiment and purification and isolation of the serine obtained, the biotechnologist observed that 0.1g of the product sample from serinase A reaction dissolved in 1mL of water, measured in a cell of path length 10cm, gave an optical rotation of +1.2 degrees, while a similar weight of sample from the serinase B reaction in the same cell gave an optical rotation of +1.4 degrees. Assuming that only serine is present in the test and reaction samples, determine: (i) the specific rotation of L-serine and D-serine? (2 Marks) (ii) the enantiomeric excess of the serinase A and serinase B reactions (4 Marks) (iii) Which of the two systems would be preferred for the production of D-serine? (2 Marks) (iv) Explain the rationale for the answer in 3b (iii) above ( 2 Marks) (Total Marks: 10 Marks)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


