Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(b) A reductive current is passed through an aqueous solution of methyl viologen dication to effect the reaction MV2++ee+MV+. The optical absorbance at 600nm(max for
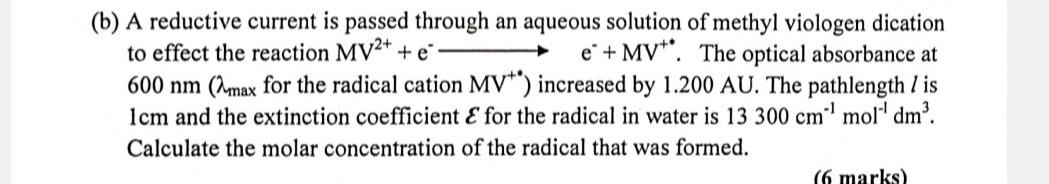
(b) A reductive current is passed through an aqueous solution of methyl viologen dication to effect the reaction MV2++ee+MV+. The optical absorbance at 600nm(max for the radical cation MV+ ) increased by 1.200AU. The pathlength l is 1cm and the extinction coefficient E for the radical in water is 13300cm1mol1dm3. Calculate the molar concentration of the radical that was formed
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


