Answered step by step
Verified Expert Solution
Question
1 Approved Answer
b. Acetylene (C2H2) may be formed from methane (CH4) by pyrolyzing-decomposing at high temperature according to the reaction: 2CH4 (g) C2H2 (g) + 3H2 (g)
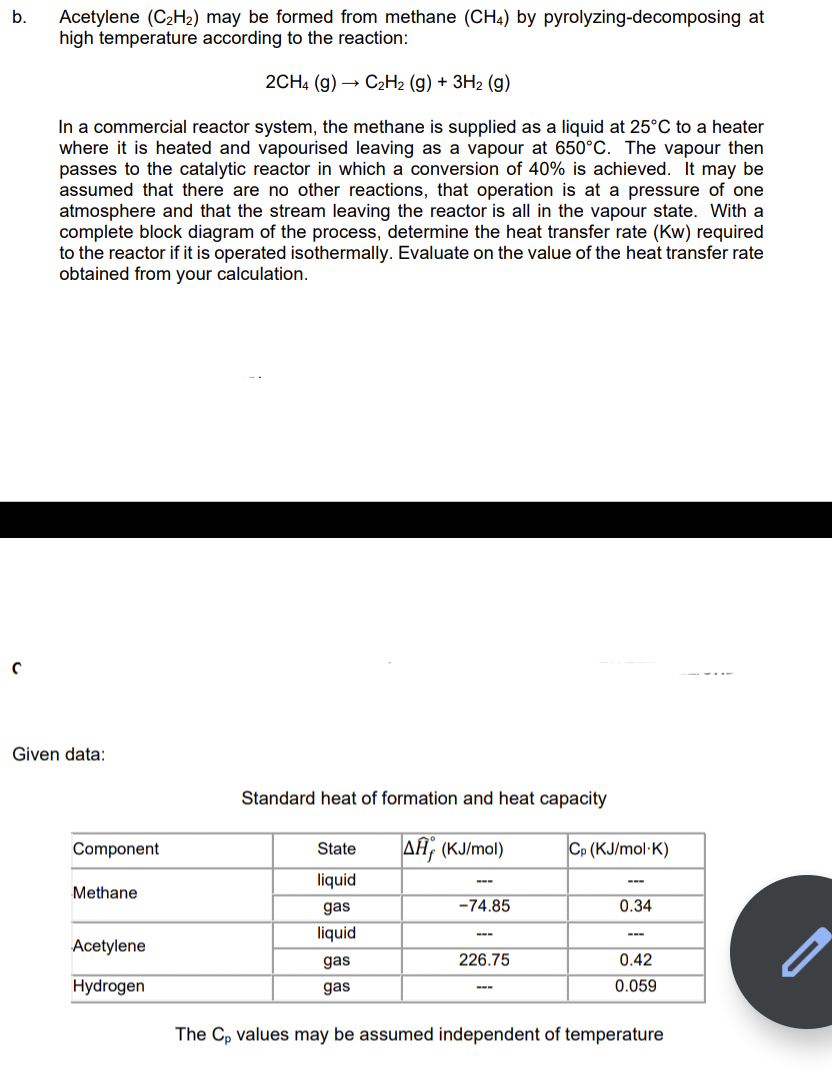
b. Acetylene (C2H2) may be formed from methane (CH4) by pyrolyzing-decomposing at high temperature according to the reaction: 2CH4 (g) C2H2 (g) + 3H2 (g) In a commercial reactor system, the methane is supplied as a liquid at 25C to a heater where it is heated and vapourised leaving as a vapour at 650C. The vapour then passes to the catalytic reactor in which a conversion of 40% is achieved. It may be assumed that there are no other reactions, that operation is at a pressure of one atmosphere and that the stream leaving the reactor is all in the vapour state. With a complete block diagram of the process, determine the heat transfer rate (Kw) required to the reactor if it is operated isothermally. Evaluate on the value of the heat transfer rate obtained from your calculation. C Given data: Standard heat of formation and heat capacity Component State A; (KJ/mol) Cp (KJ/mol K) Methane liquid gas liquid -74.85 0.34 Acetylene 226.75 gas gas 0.42 0.059 Hydrogen The Cp values may be assumed independent of temperature
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


