Question
(b) An experiment was carried out as follows: 1.5766 g of an ammonium salt was placed in a beaker and 60 mL of 1.182
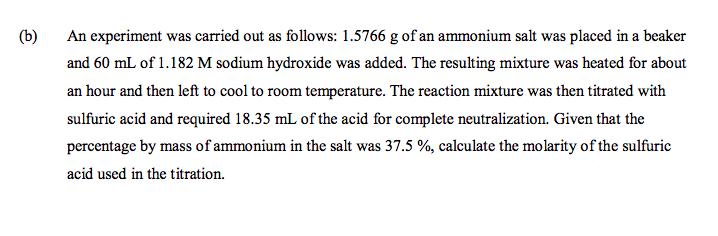
(b) An experiment was carried out as follows: 1.5766 g of an ammonium salt was placed in a beaker and 60 mL of 1.182 M sodium hydroxide was added. The resulting mixture was heated for about an hour and then left to cool to room temperature. The reaction mixture was then titrated with sulfuric acid and required 18.35 mL of the acid for complete neutralization. Given that the percentage by mass of ammonium in the salt was 37.5 %, calculate the molarity of the sulfuric acid used in the titration.
Step by Step Solution
3.43 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Statistics The Exploration & Analysis Of Data
Authors: Roxy Peck, Jay L. Devore
7th Edition
0840058012, 978-0840058010
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



