Question
b) Based on the balanced equation: 2A + B+3C AB -> [A]o (mol/L) [B]o (mol/L) [C]o (mol/L) Initial Rate (mol/L s) 1.00 1.00 1.00
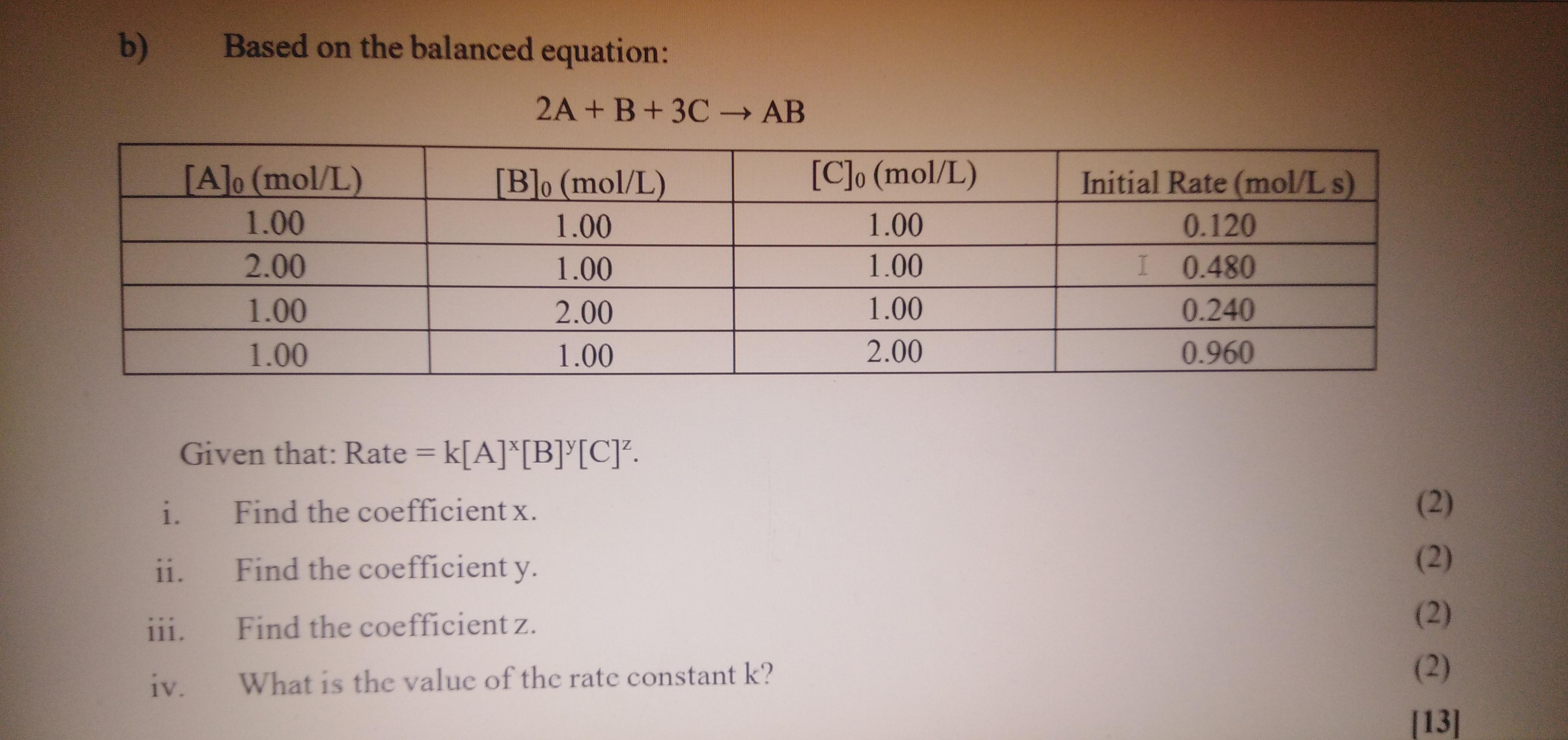
b) Based on the balanced equation: 2A + B+3C AB -> [A]o (mol/L) [B]o (mol/L) [C]o (mol/L) Initial Rate (mol/L s) 1.00 1.00 1.00 0.120 2.00 1.00 1.00 I 0.480 1.00 2.00 1.00 0.240 1.00 1.00 2.00 0.960 Given that: Rate = k[A]*[B]*[C]. %3D |3D i. Find the coefficient x. (2) ii. Find the coefficient y. (2) iii. Find the coefficient z. (2) (2) iv. What is the value of the rate constant k? [13]
Step by Step Solution
3.44 Rating (147 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Forensic And Investigative Accounting
Authors: G. Stevenson Smith D. Larry Crumbley, Edmund D. Fenton
10th Edition
0808056301, 9780808056300
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



