Answered step by step
Verified Expert Solution
Question
1 Approved Answer
B. Calculate AHsolution (Na2S04 10 H20). (0.4) oehoga ded C. Calculate your experimental value for AHnydration (NazSO4) applying Hess's law. Show the addition of

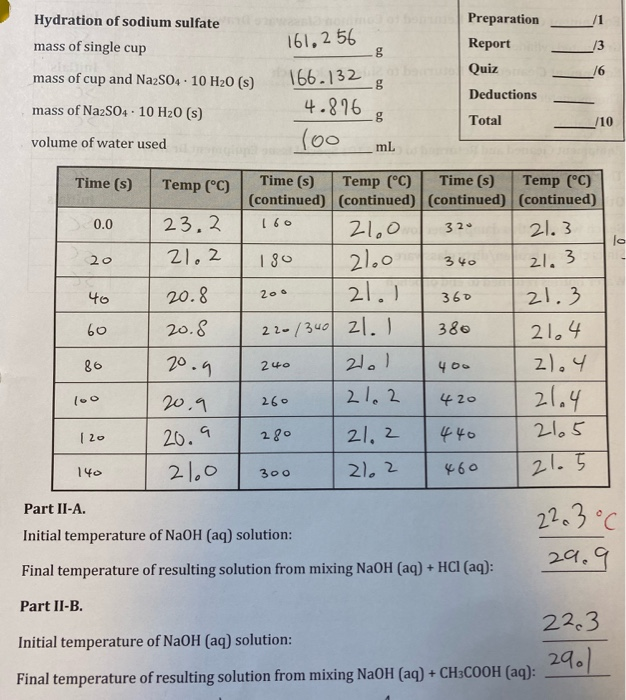
B. Calculate AHsolution (Na2S04 10 H20). (0.4) oehoga ded C. Calculate your experimental value for AHnydration (NazSO4) applying Hess's law. Show the addition of the appropriate net ionic equations used to obtain AHhydration (NazSO4). (0.5) Hydration of sodium sulfate Preparation 161,2 56 g mass of single cup Report 13 Quiz 16 mass of cup and NazS04 10 H20 (s) 166.132 g Deductions mass of NazS04 10 H20 (s) 4.876 Total /10 (00 volume of water used mL Time (s) Time (s) Temp (C) (continued) (continued) (continued) (continued) 21. 3 Time (s) Temp (C) Temp (C) 0.0 23,2 160 21.0 320 21.0 21.1 21.2 180 20 21.3 340 20.8 21.3 40 200 360 20.8 22-/340 21. 380 21,4 60 20.9 21.1 2).4 86 240 400 21.4 2165 20.9 21.2 420 l00 260 21, 2 440 20.9 280 |20 21.0 21, 2 21.5 140 300 460 Part II-A. 22.3C Initial temperature of NaOH (aq) solution: 29.9 Final temperature of resulting solution from mixing NaOH (aq) + HCI (aq): Part II-B. 22.3 Initial temperature of NaOH (aq) solution: 29.1 Final temperature of resulting solution from mixing NaOH (aq) + CH3COOH (aq):
Step by Step Solution
★★★★★
3.34 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


