Answered step by step
Verified Expert Solution
Question
1 Approved Answer
b Course Contents Prelab 1 - Rocrystallation and Melting Point analysis 95.problem In a recrystallization process, the impurities should either be completely soluble in the
b 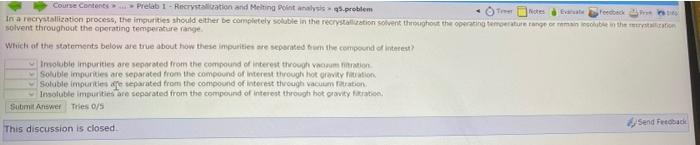
Course Contents Prelab 1 - Rocrystallation and Melting Point analysis 95.problem In a recrystallization process, the impurities should either be completely soluble in the restauration solvent throughout the operating temperature range in the solvent throughout the operating temperature range Which of the statements below are true about how these impurities are separated in the compound of interest Inouble impurities are separated from the compound of interest through common Soluble impurities are separated from the compound of interest through hot vition Soluble impurities are separated from the compound of interest through vacuumatration insoluble impurities are separated from the compound of interest through hot gravity fatration Submit Answer Tries 0/5 Send Feedback This discussion is closed Course Contents Prelab 1 - Rocrystallation and Melting Point analysis 95.problem In a recrystallization process, the impurities should either be completely soluble in the restauration solvent throughout the operating temperature range in the solvent throughout the operating temperature range Which of the statements below are true about how these impurities are separated in the compound of interest Inouble impurities are separated from the compound of interest through common Soluble impurities are separated from the compound of interest through hot vition Soluble impurities are separated from the compound of interest through vacuumatration insoluble impurities are separated from the compound of interest through hot gravity fatration Submit Answer Tries 0/5 Send Feedback This discussion is closed 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


