Answered step by step
Verified Expert Solution
Question
1 Approved Answer
B. Density of Liquid (sugar solutions) Table 1: Calculate the densities of three sugar solutions of known concentrations Sugar solution 1 Sugar solution 2 Sugar
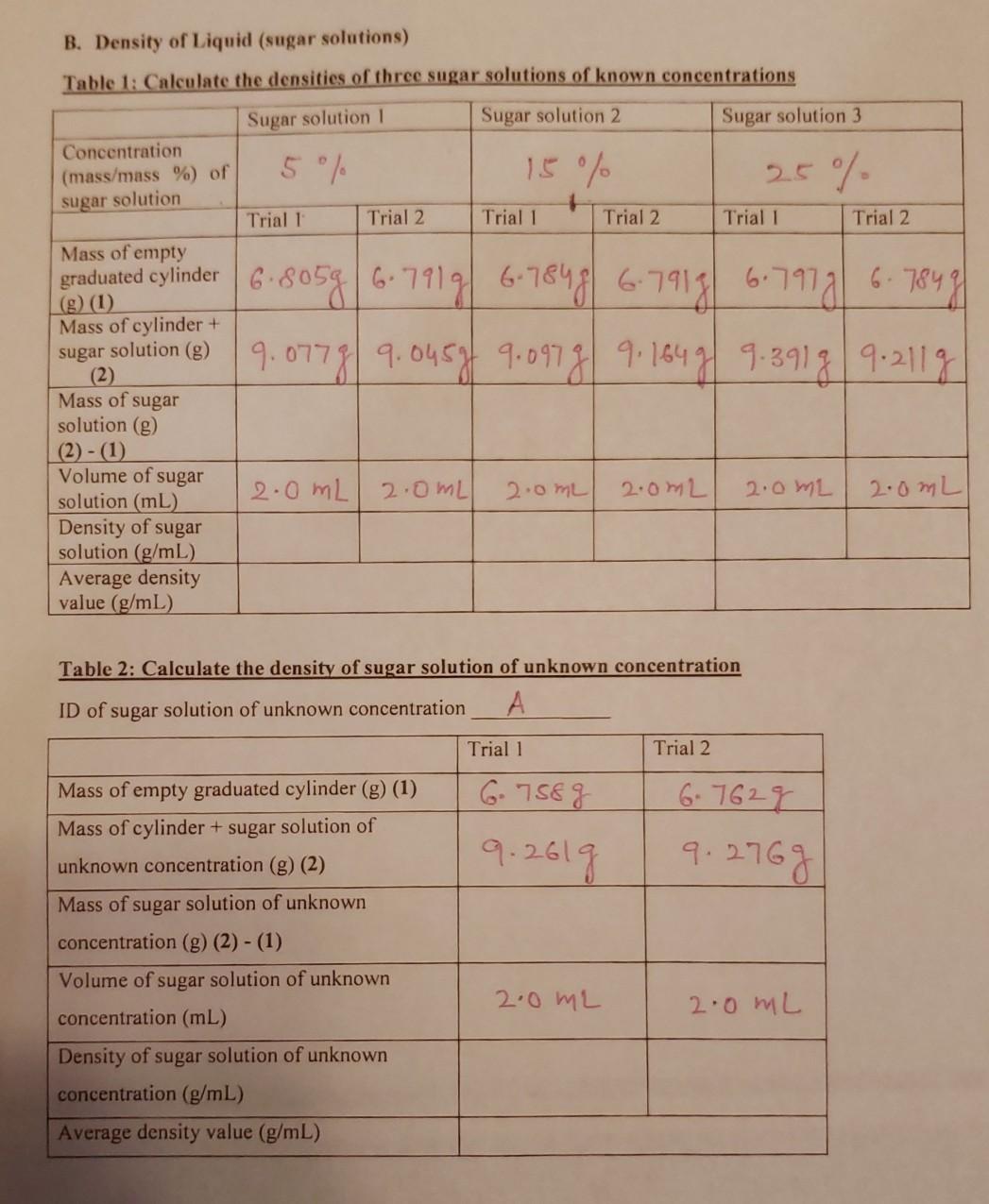
B. Density of Liquid (sugar solutions) Table 1: Calculate the densities of three sugar solutions of known concentrations Sugar solution 1 Sugar solution 2 Sugar solution 3 Concentration (mass/mass %) of 5 / 15 % 25% sugar solution Trial 1 Trial 2 Trial 1 Trial 2 Trial 1 Trial 2 Mass of empty (g) (1) Mass of cylinder + sugar solution (g) graduated cylinder 6.80586.7919 6.78486-7913 6.7918 6.7847 9.0778/ 9.045gf 9.097g| 9. 1649/ 9.391g|9:2119 2.0 mL 2.0mL) 2.0mL 2.0m2 Mass of sugar solution (g) (2) -(1) Volume of sugar solution (mL) Density of sugar solution (g/mL) Average density value (g/mL) 2.0 ML 2.0mL Table 2: Calculate the density of sugar solution of unknown concentration ID of sugar solution of unknown concentration Trial 1 Trial 2 6 758 g 6.7622 9.276g 9.261g Mass of empty graduated cylinder (g) (1) Mass of cylinder + sugar solution of unknown concentration (g) (2) Mass of sugar solution of unknown concentration (g) (2) - (1) Volume of sugar solution of unknown concentration (mL) Density of sugar solution of unknown concentration (g/mL) Average density value (g/mL) 2.0 ML 2.0 ML
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


