Answered step by step
Verified Expert Solution
Question
1 Approved Answer
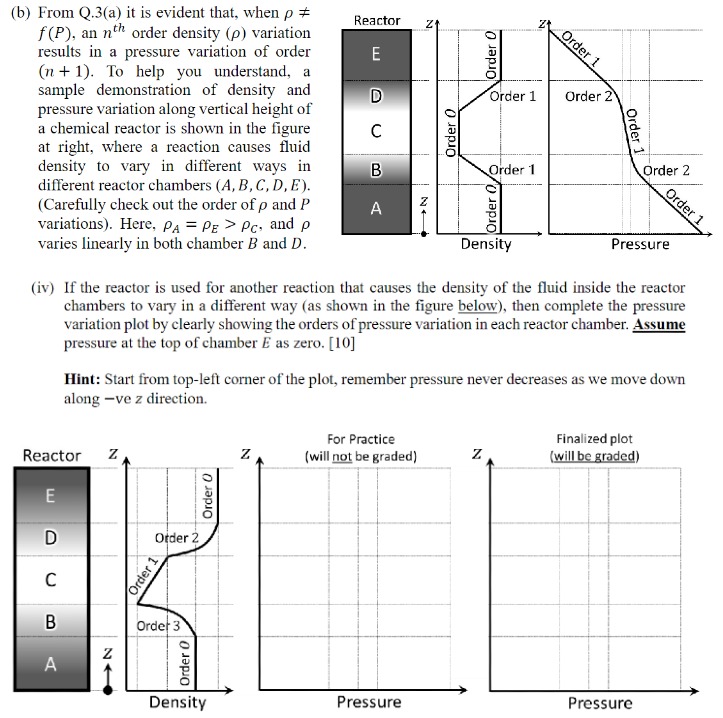
( b ) From Q . 3 ( a ) it is evident that, when f ( P ) , an n t h order
b From Qa it is evident that, when
an order density variation
results in a pressure variation of order
To help you understand, a
sample demonstration of density and
pressure variation along vertical height of
a chemical reactor is shown in the figure
at right, where a reaction causes fluid
density to vary in different ways in
different reactor chambers
Carefully check out the order of and
variations Here, and
varies linearly in both chamber and
iv If the reactor is used for another reaction that causes the density of the fluid inside the reactor
chambers to vary in a different way as shown in the figure below then complete the pressure
variation plot by clearly showing the orders of pressure variation in each reactor chamber. Assume
pressure at the top of chamber as zero.
Hint: Start from topleft corner of the plot, remember pressure never decreases as we move down
along ve direction.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


