Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. You are using a three-electrode setup with an Ag/AgCl reference electrode containing 0.1 M KCI. The reaction you are studying has a standard
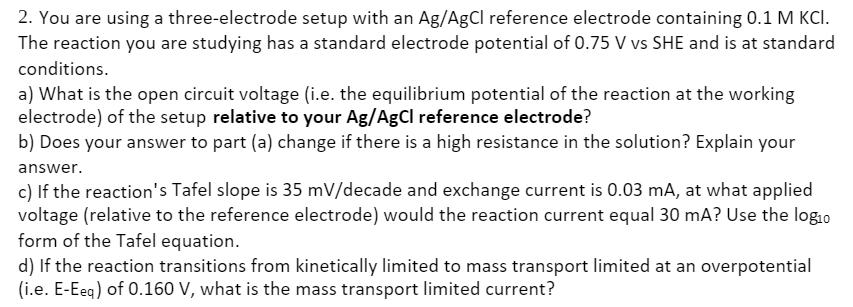
2. You are using a three-electrode setup with an Ag/AgCl reference electrode containing 0.1 M KCI. The reaction you are studying has a standard electrode potential of 0.75 V vs SHE and is at standard conditions. a) What is the open circuit voltage (i.e. the equilibrium potential of the reaction at the working electrode) of the setup relative to your Ag/AgCl reference electrode? b) Does your answer to part (a) change if there is a high resistance in the solution? Explain your answer. c) If the reaction's Tafel slope is 35 mV/decade and exchange current is 0.03 mA, at what applied voltage (relative to the reference electrode) would the reaction current equal 30 mA? Use the logio form of the Tafel equation. d) If the reaction transitions from kinetically limited to mass transport limited at an overpotential (i.e. E-Eeq) of 0.160 V, what is the mass transport limited current?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


