Answered step by step
Verified Expert Solution
Question
1 Approved Answer
B. Silver - Copper Cu(s), CuCl2 (1.0 M) || Ag(s), AgNO3 (1.0 M) 1. Draw the schematic of the electrochemical cell that you created including
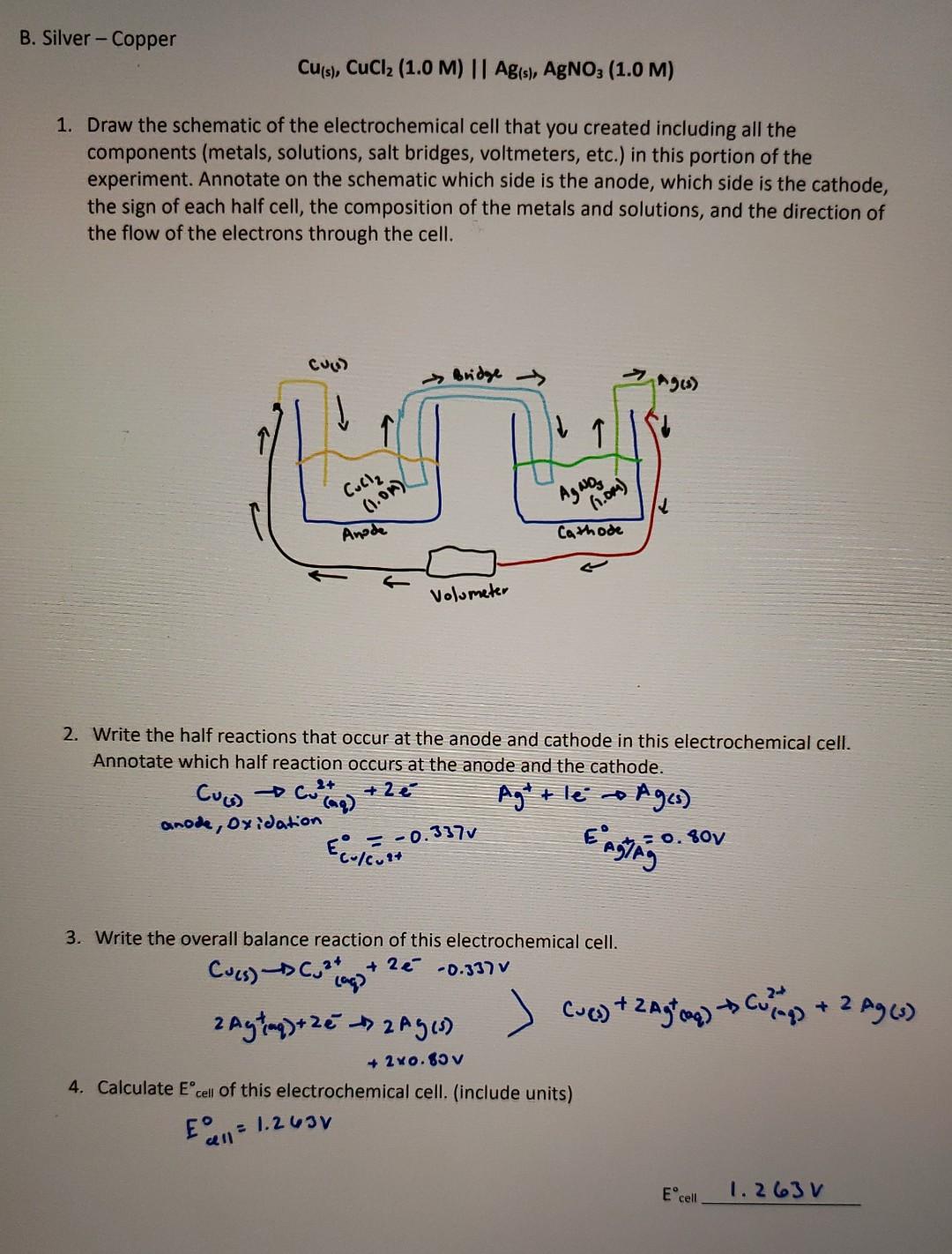
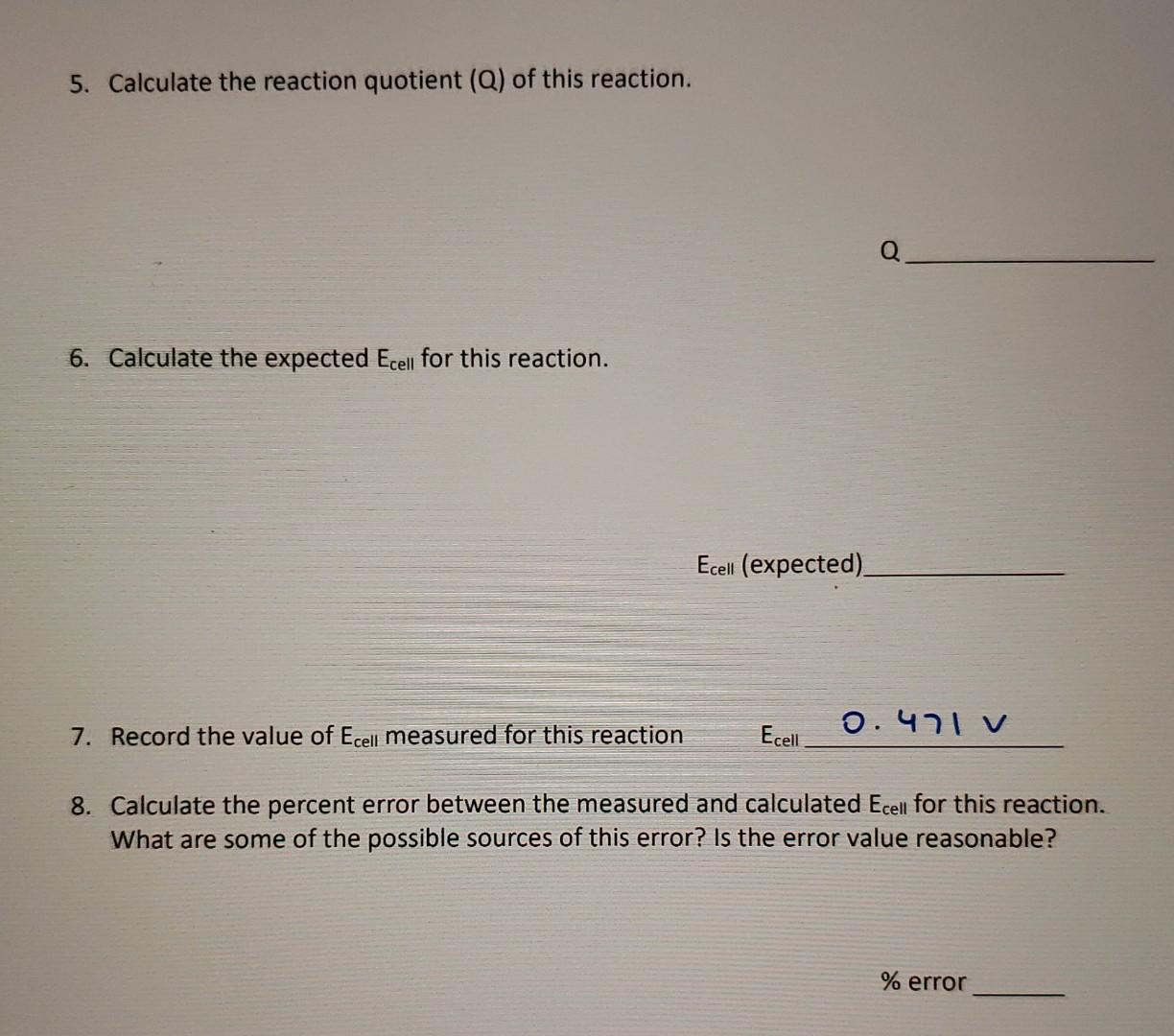
B. Silver - Copper Cu(s), CuCl2 (1.0 M) || Ag(s), AgNO3 (1.0 M) 1. Draw the schematic of the electrochemical cell that you created including all the components (metals, solutions, salt bridges, voltmeters, etc.) in this portion of the experiment. Annotate on the schematic which side is the anode, which side is the cathode, the sign of each half cell, the composition of the metals and solutions, and the direction of the flow of the electrons through the cell. CUAD Bridge Agen Culle (1.01) AgNO3 6.0m) Anode Cathode Volumeter 2. Write the half reactions that occur at the anode and cathode in this electrochemical cell. Annotate which half reaction occurs at the anode and the cathode. + 2 Cus). 2+ collag anode, oxidation Agtt le a Ages) Engang -0.337v 0.807 Ecolevat 3. Write the overall balance reaction of this electrochemical cell. Cucs).** +2 -0.337 v lag? Cuevy + zagog) to cumps + 2 Agw 2 Aytinq) + 2e Ages) + 2x0.85v 4. Calculate Ecell of this electrochemical cell. (include units) EEN = 1.263V Ecell 1.263 V 5. Calculate the reaction quotient (Q) of this reaction. Q 6. Calculate the expected Ecell for this reaction. Ecell (expected). 7. Record the value of Ecell measured for this reaction Ecell O.471 v 8. Calculate the percent error between the measured and calculated Ecell for this reaction. What are some of the possible ces of this error? Is the error value reaso % error
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


