Question
(B) Some final year Chemical Engineering students conducted experiments to explore the use of pyrolyzed Spent Bleaching Earth (SBE) as low cost, flexible, sustainable and
(B) Some final year Chemical Engineering students conducted experiments to explore the use of pyrolyzed Spent Bleaching Earth (SBE) as low cost, flexible, sustainable and environmentally friendly adsorbent, or the removal of recalcitrant organic pollutants in aqueous matrices. Methylene blue dye was used as the model pollutant. As part of their studies, the students sought to identify which adsorption isotherm model best fits the data obtained.
(a) The table below presents a summary of the experimental data that was obtained upon varying the initial concentration (Ci) and determining the equilibrium concentration (Ce) after contacting the 10g/L of spent bleaching earth for 24 hours.
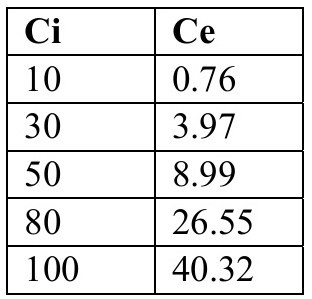
(i) Using this data, evaluate which adsorption isotherm model between, Langmuir and Freundlich best fits the data. (12) NB. All relevant graphs and constants obtained should be reported.
(ii) Based on the answer obtained in (i) comment on the nature of the expected interaction between the adsorbate and adsorbent.
\begin{tabular}{|l|l|} \hline Ci & Ce \\ \hline 10 & 0.76 \\ \hline 30 & 3.97 \\ \hline 50 & 8.99 \\ \hline 80 & 26.55 \\ \hline 100 & 40.32 \\ \hline \end{tabular}Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


