Question: b. Two parallel reactions are taking place in a reactor system. Reaction 1: A+B-R Reaction 2: R+B-S r = k, CSc.5 r2 = kz CRESCE
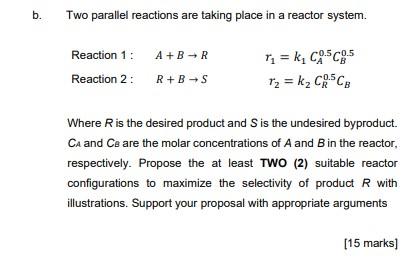
b. Two parallel reactions are taking place in a reactor system. Reaction 1: A+B-R Reaction 2: R+B-S r = k, CSc.5 r2 = kz CRESCE Where is the desired product and is the undesired byproduct. CA and Ce are the molar concentrations of A and B in the reactor, respectively. Propose the at least TWO (2) suitable reactor configurations to maximize the selectivity of product R with illustrations. Support your proposal with appropriate arguments [15 marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


